Chapter 12 Glucosamine Chondroitin
 Initial Examination
Initial Examination
Employment: John is a retired engineer. His wife is also retired.
Medications: Captopril, hydrochlorothiazide, na-proxen
Laboratory: Normal except for PSA 6 mg/ml
Radiographs: Narrowing of joint space and osteophyte formation
Integumentary: Mild swelling bilaterally
Cardiopulmonary: BP 143/80, HR 72
Function: Limited by pain rating of 2/10 at rest, increasing to 6/10 with elevations and ambulation
INVESTIGATING THE LITERATURE
Preliminary Reading
Glucosamine, as the name implies, is an amino glucose. It is a component of cartilage proteoglycans and essential for the synthesis of glycoproteins, glycolipids, and glycosaminoglycans in the human body.1 Supplements of glucosamine are derived from exoskeletons of marine life or are manufactured synthetically. Glucosamine sulfate is the form most widely available and used in clinical trials.
Glucosamine is thought to stimulate the metabolism of chondrocytes of articular cartilage and the synovial cells of synovial tissue and is purported to rebuild damaged cartilage and promote antiinflammatory and analgesic activity.1,2 By these actions, glucosamine may help provide relief of symptoms and slow the progression of OA.
Chondroitin, as chondroitin sulfate, is classified as a glucosaminoglycan, which is composed of glucuronic acid and galactosamine.1 Supplements of chondroitin are derived from shark or bovine cartilage.3 Chondroitin plays a role in the formation of joint matrix structures in mammals and may protect cartilage degradation in OA.1,3
The abundance of supplement manufacturers and the relative ease of getting a product to market are the result of the passage of the Dietary Supplement Health and Education Act of 1994 (DSHEA, pronounced “De-shay”). DSHEA was an amendment to the Food, Drug, and Cosmetic Act, which made dietary supplements and dietary supplement ingredients exempt from the regulations that apply to food and drugs.4 The DSHEA stipulated that the dietary supplement must include a disclaimer statement on the product label that the product has not been evaluated by the Food and Drug Administration (FDA); however, it conveys no judgment from an authoritative source that the product is effective or safe. At times the allowable “structure-function” claims on dietary supplements are confusing to consumers in that no regulation exists regarding the claims to health and wellness.
In practice, if a person wishes to use dietary supplements, the best choice is to buy from a reputable manufacturer and look for voluntary analyses by independent agencies. If a product undergoes and passes quality standards by an independent agency, the product can bear the seal of approval. A sample of four popular agencies and their corresponding seals are depicted in Table 12-1.
Table 12-1 Sample of Independent Agencies Assessing the Quality of Dietary Supplements
| AGENCY | URL | QUALITY SEAL |
|---|---|---|
| ConsumerLab | http://www.consumerlab.com | |
| NSF International | http://www.nsf.org | |
| Shuster Labs | http://www.shusterlabs.com | Technically Advanced Quality Assurance (TAQA) program |
| US Pharmacopia | http://www.usp.org |
Searching the Databases
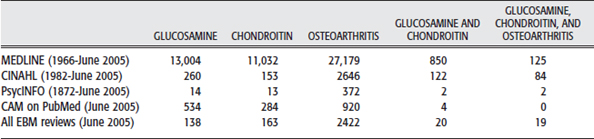
The initial search strategy for finding research on the use of glucosamine and chondroitin for OA is conducted on MEDLINE. A general search is done initially using the term osteoarthritis and including all the subcategories. Likewise, the terms glucosamine and then chondroitin are searched. Because this evaluation is to include the use of glucosamine and chondroitin, the terms are combined. Finally the terms glucosamine and chondroitin were combined with osteoarthritis. This search strategy is repeated in CINAHL, PsycINFO, CAM on PubMed, and all evidence-based reviews. Search results then may be further refined to return articles only with human participants and in the English language. As desired the search also can be limited to review articles, those available with full text online, or most recent publication dates. For purposes of this search, articles are limited to evidence-based reviews. See results of the search in Table 12-2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Incorporating CAM into the Plan of Care
Incorporating CAM into the Plan of Care