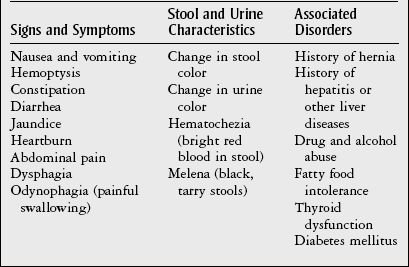
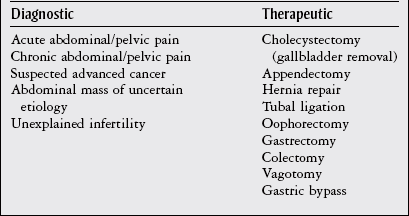
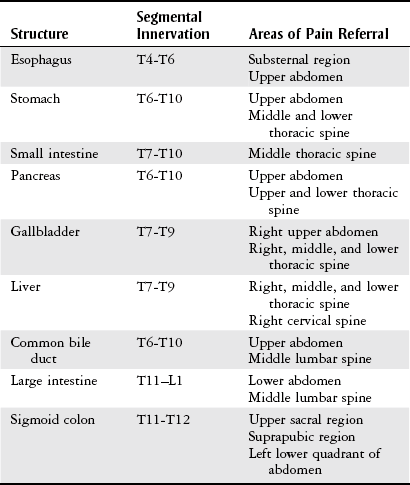
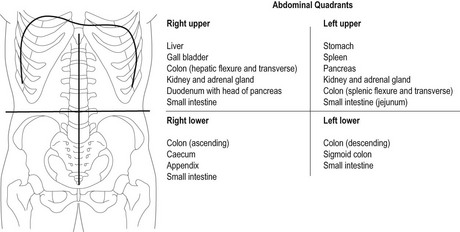
Chapter 8 The objectives of this chapter are to provide the following: 1 An understanding of the structure and function of the gastrointestinal (GI) system 2 Information on the clinical evaluation of the GI system, including physical examination and diagnostic studies 3 An overview of the various diseases and disorders of the GI system 4 Information on the management of GI disorders, including pharmacologic therapy and surgical procedures 5 Guidelines for physical therapy intervention in patients with GI diseases and disorders • Primary Prevention/Risk Reduction for Skeletal Demineralization: 4A • Impaired Aerobic Capacity/Endurance Associated with Deconditioning: 6B • Primary Prevention/Risk Reduction for Integumentary Disorders: 7A Please refer to Appendix A for a complete list of the preferred practice patterns, as individual patient conditions are highly variable and other practice patterns may be applicable. Disorders of the gastrointestinal (GI) system can have numerous effects on the body, such as decreased nutrition, anemia, and fluid imbalances. These consequences may, in turn, affect the activity tolerance of a patient, which will ultimately influence many physical therapy interventions. In addition, physical therapists must be aware of pain referral patterns from the GI system that may mimic musculoskeletal symptoms (Table 8-1). TABLE 8-1 Gastrointestinal System Pain Referral Patterns From Boissonault WG, Bass C: Pathological origins of trunk and neck pain: part I. Pelvic and abdominal visceral disorders, J Orthop Sports Phys Ther 12:194, 1990. The basic structure of the GI system is shown in Figure 8-1, with the primary and accessory organs of digestion and their respective functions described in Tables 8-2 and 8-3. TABLE 8-2 Structure and Function of the Primary Organs of Digestion Data from Scanlon JC, Sanders T, editors: Essentials of anatomy and physiology, ed 2, Philadelphia, 1993, FA Davis, p 362; Patton KT: Anatomy & physiology (with media), ed 7, St Louis, 2009, Mosby, pp 837-863. TABLE 8-3 Structure and Function of the Accessory Organs of Digestion *The spleen is not part of the gastrointestinal system but is located near other gastrointestinal components in the abdominal cavity. Data from Scanlon JC, Sanders T, editors: Essentials of anatomy and physiology, ed 2, Philadelphia, 1993, FA Davis; Patton KT: Anatomy & physiology (with media), ed 7, St Louis, 2009, Mosby, pp 837-863. Before performing the physical examination, the presence or absence of items related to GI pathology (Box 8-1) is ascertained through patient interview, questionnaire completion, or chart review. Please see Chapter 2 for a description of the general medical record review. Figure 8-2 demonstrates the abdominal regions associated with organ location. During inspection, the physical therapist should note asymmetries in size and shape in each quadrant, umbilicus appearance, and presence of abdominal scars indicative of previous abdominal procedures or trauma.1 FIGURE 8-2 The four abdominal quadrants, showing the viscera found in each. (From Palastanga N, Soames RW: Anatomy and human movement: structure and function, ed 6, Edinburgh, 2012, Churchill Livingstone.) The abdomen is auscultated for the presence or absence of bowel sounds and bruits (murmurs) to help evaluate gastric motility and vascular flow, respectively. Bowel sounds can be altered postoperatively, as well as in cases of diarrhea, intestinal obstruction, paralytic ileus, and peritonitis. The presence of bruits over the aorta or the renal, iliac, or femoral arteries may be indicative of vascular disease.3 Mediate percussion is used to evaluate liver and spleen size and borders, as well as to identify ascitic fluid, solid- or fluid-filled masses, and air in the stomach and bowel.3 The technique for mediate percussion is described in the Physical Examination section of Chapter 4. Light palpation and deep palpation are used to identify abdominal tenderness, muscular resistance, and superficial organs and masses. The presence of rebound tenderness (i.e., abdominal pain worsened by a quick release of palpatory pressure) is an indication of possible peritoneal irritation and requires immediate medical attention. Muscle guarding during palpation may also indicate a protective mechanism for underlying visceral pathology.3,4 Discussion of the diagnostic evaluation for the GI system is divided into (1) the examination of the GI tract and (2) the examination of the hepatic, biliary, pancreatic, and splenic systems. Examination of the GI tract includes the esophagus, stomach, and intestines (small and large). Table 8-4 summarizes the laboratory tests used to measure functional aspects of secretion, digestion, absorption, and elimination within the GI tract.5–12 Table 8-5 summarizes diagnostic procedures used to visualize the GI tract. TABLE 8-4 Laboratory Tests for the Gastrointestinal System* *Words or abbreviations in parentheses are synonyms for the test names. Data from Pagana KD, Pagana TJ: Mosby’s diagnostic and laboratory test reference, ed 9, St Louis, 2009, Mosby; Lab Tests Online: http://labtestsonline.org. Accessed July 12, 2012. TABLE 8-5 Diagnostic Procedures for the Gastrointestinal (GI) System* *Words or abbreviations in parentheses are synonyms for the test names. Data from Malarkey LM, McMorrow ME, editors: Nurse’s manual of laboratory tests and diagnostic procedures, Philadelphia, 2000, Saunders, pp 432-468; Pagana KD, Pagana TJ: Mosby’s diagnostic and laboratory test reference, ed 9, St Louis, 2009, Mosby; Andreoli TE, Benjamin IJ, Griggs RC et al: Andreoli and Carpenter’s Cecil essentials of medicine, ed 8, Philadelphia, 2010, Saunders. Examination of the hepatic (liver), biliary (gallbladder and cystic ducts), pancreatic, and splenic systems involves numerous laboratory tests and diagnostic procedures, which are often performed concurrently to fully delineate the etiology of a patient’s clinical presentation. Because of the common location of these organs and shared access to the biliary tree, disease or dysfunction in one organ can often extend into the other organs.13,14 Hepatocellular disease results in cellular damage in the liver, which causes increased levels of the following enzymes: aspartate aminotransferase (AST; previously called serum glutamic-oxaloacetic transaminase), alanine aminotransferase (ALT; previously called serum glutamate pyruvate transaminase), and lactate dehydrogenase (LD).14,15 Hepatocellular dysfunction can be identified when bilirubin levels are elevated or when clotting times are increased (denoted by an increased prothrombin time and the international normalized ratio [INR]). The liver produces clotting factors, and therefore an increased prothrombin time or INR implicates impaired production of coagulation factors. Refer to Chapter 7 for more details on coagulation tests. Cholestasis is the impairment of bile flow from the liver to the duodenum and results in elevations of the following serum enzymes: alkaline phosphatase (ALP), aspartate transaminase (previously known as γ-glutamine-oxaloacetic transaminase or γ-glutamyl transpeptidase), and 5′-nucleotidase.14,15 Table 8-6 summarizes the laboratory tests performed to measure hepatic, biliary, and pancreatic function. Table 8-7 summarizes the diagnostic procedures performed to visualize these organs. TABLE 8-6 Laboratory Tests for the Hepatic, Biliary, and Pancreatic Systems* *Words or abbreviations in parentheses are synonyms for the test names. Data from Knight JA: Liver function tests: their role in the diagnosis of hepatobiliary diseases, J Infus Nurs 28(2):108-117, 2005; Parad RB, Comeau AM, Dorkin HL et al: Sweat testing infants detected by cystic fibrosis newborn screening, J Pediatr 147(3 Supp 1):S69-S72, 2005; Davies JC: New tests for cystic fibrosis, Paediatr Respir Rev 7(S1:S141-S143, 2006; Pagana KD, Pagana TJ: Mosby’s diagnostic and laboratory test reference, ed 9, St Louis, 2009, Mosby; Lab Tests Online: http://labtestsonline.org. Accessed July 12, 2012. TABLE 8-7 Diagnostic Procedures for the Hepatic, Biliary, Pancreatic, and Splenic Systems* *Words or abbreviations in parentheses are synonyms for the test names. Data from Knight JA: Liver function tests: their role in the diagnosis of hepatobiliary diseases, J Infus Nurs 28(2):108-117, 2005; Parad RB, Comeau AM, Dorkin HL et al: Sweat testing infants detected by cystic fibrosis newborn screening, J Pediatr 147(3 Supp 1):S69-S72, 2005; Davies JC: New tests for cystic fibrosis, Paediatr Respir Rev 7(S1:S141-S143, 2006; Fulcher AS: MRCP and ERCP in the diagnosis of common bile duct stones, Gastrointest Endosc 56(6 Supp 1):S178-S182, 2002; Taylor ACF, Little AF, Hennessy OF et al: Prospective assessment of magnetic resonance cholangiopancreatography for noninvasive imaging of the biliary tree, Gastrointest Endosc 55(1):17-22, 2002; Miller AH, Pepe PE, Brockman CR et al: ED ultrasound in hepatobiliary disease, J Emerg Med 30(1):69-74, 2006; Kalimi R, Gecelter GR, Caplin D et al: Diagnosis of acute cholecystitis: sensitivity of sonography, cholescintigraphy, and combined sonography-cholescintigraphy, J Am Coll Surg 193(6):609-613, 2001; Pagana KD, Pagana TJ: Mosby’s diagnostic and laboratory test reference, ed 9, St Louis, 2009, Mosby; Ferri’s clinical advisor 2013, St Louis, 2012, Mosby. Laparoscopy is the insertion of a laparoscope (a fiberoptic tube) into the abdominal cavity through a small incision in the periumbilical area. To perform this procedure, a local anesthetic is given, and gas (i.e., nitric oxide or carbon dioxide) is infused into the abdominal cavity to allow better visualization and manipulation of the scope.16 Box 8-2 describes the diagnostic and therapeutic interventions that may be performed with a laparoscope. The use of MRI of the GI system is primarily indicated for imaging of the liver for hepatic tumors, iron overload, and hepatic and portal venous occlusion.17 Otherwise, computed tomography scans are preferred for the visualization of other abdominal organs.18 Good success, however, has been reported recently in using MRI for defining tissue borders for managing and resecting colorectal tumors.19,20 MRI has also been successful in helping to delineate the etiology of cirrhosis between alcohol abuse and viral hepatitis.21 PET is the use of positively charged ions and computer technology to create color images of organs and their functions. Combination technology using both PET and CT scanning is also available. Clinical uses of PET for the GI system include evaluation of pancreatic function and GI cancer.22,23 Dysphagia, or difficulty swallowing, can occur from various etiologies (Table 8-8) and is generally classified by its location as either oropharyngeal dysphagia occurring in the pharynx or upper esophagus, or esophageal dysphagia occurring in the esophageal body or lower esophageal sphincter.24 TABLE 8-8 Classification and Possible Etiologies of Dysphagia Data from Satpathy HK: Dysphagia. In Ferri F, editor: Ferri’s clinical advisor 2013, St Louis, 2012, Mosby. The following characteristics of dysphagia should also be noted to aid in the diagnosis: • Does it occur with ingestion of solids, liquids, or both? • Is it intermittent, constant, or progressive? • Does the patient complain of regurgitation/reflux or coughing while eating? • The location at which the food becomes stuck should also be noted.24 Diagnosis can be established with imaging studies such as video fluoroscopy, modified barium swallow study, endoscopy, CT, and MRI. The primary goal of treatment includes airway protection and maintenance of nutrition with specific strategies employed once a definitive etiology is established.25 Poor esophageal motility from neuromuscular dysfunction can result in dysphagia, chest pain, or heartburn. Achalasia, GERD, and distal esophageal spasm (DES) are the most common causes of primary esophageal motility disorders.26 Achalasia is a neuromuscular disorder of esophageal motility characterized by impaired lower esophageal sphincter (LES) relaxation and aperistalsis (absence of peristalsis) in the smooth muscle of the esophagus. Innervation to both the LES and the smooth muscle is thought to be disrupted, resulting in loss of motility. In addition to the aforementioned symptoms, clinical manifestations may include chest pain, regurgitation, hiccups, halitosis, weight loss, and aspiration pneumonia. All patients with achalasia will have solid food dysphagia with variable degrees of liquid aphasia. Treatment is aimed at compensating for poor esophageal emptying by reducing LES pressure with pharmacologic therapy, forceful dilation, or surgical myotomy.26,27 Distal esophageal spasm is characterized by the occurrence of normal peristalsis with intermittent nonperistaltic, simultaneous contractions of the body of the esophagus. Clinical manifestations include intermittent chest pain with or without eating that may be similar in character to angina, described as squeezing or crushing in nature, radiating to the jaw, neck, arms, or midline of the back.26 The etiology is unknown, and management is directed at smooth muscle relaxation with pharmacologic agents along with behavior modification and biofeedback.26,28 GERD is characterized by gastric acid backflow into the esophagus as a result of an incompetent lower esophageal sphincter. Clinical manifestations include complaints of heartburn (especially with sour or bitter regurgitation), nausea, gagging, cough, or hoarseness. Two episodes of heartburn and/or complications from regurgitation per week defines the presence of GERD.29 Although the exact etiology of GERD is unknown, tobacco abuse, along with the consumption of alcohol, coffee, peppermint, or chocolate, has been associated with this inappropriate relaxation. Esophageal and gastric motility disorders may also contribute to GERD, as may hiatal hernia and/or obesity. Gastroesophageal reflux is a strong predisposing factor for developing esophageal adenocarcinoma. Depending on the severity of GERD, treatment can range from dietary modifications (avoid risk factors and eat small frequent meals in an upright position) to weight reduction, antacids, proton pump inhibitors, H2 blockers, or surgery.30,31 Advancements in Nissen fundoplication have shown good long-term outcomes in improving GERD symptoms.29,32 Barrett’s esophagus is a condition in which columnar epithelium replaces the normal stratified squamous mucosa of the distal esophagus. According to the American Gastrointestinal Association, “intestinal metaplasia is required for the diagnosis of Barrett’s esophagus as intestinal metaplasia is the only type of esophageal columnar epithelium that is clearly associated with malignancy.”33 Barrett’s esophagus is generally associated with chronic GERD. The mechanism of cellular metaplasia is thought to occur from chronic inflammatory injury from acid and pepsin that refluxes from the stomach into the distal esophagus.34,35 Barrett’s esophagus is also a strong predisposing factor for developing esophageal adenocarcinoma, the incidence of which rose in the 1990s.35–37 Associated signs and symptoms include dysphagia, esophagitis, ulceration, perforations, strictures, bleeding, or adenocarcinoma.35 Treatment for Barrett’s esophagus includes controlling symptoms of GERD and healing reflux esophagitis. For patients with confirmed high-grade dysplasia within Barrett’s esophagus, endoscopic eradication therapy with radiofrequency ablation, photodynamic therapy, or endoscopic mucosal resection is recommended.33
Gastrointestinal System
Preferred Practice Patterns
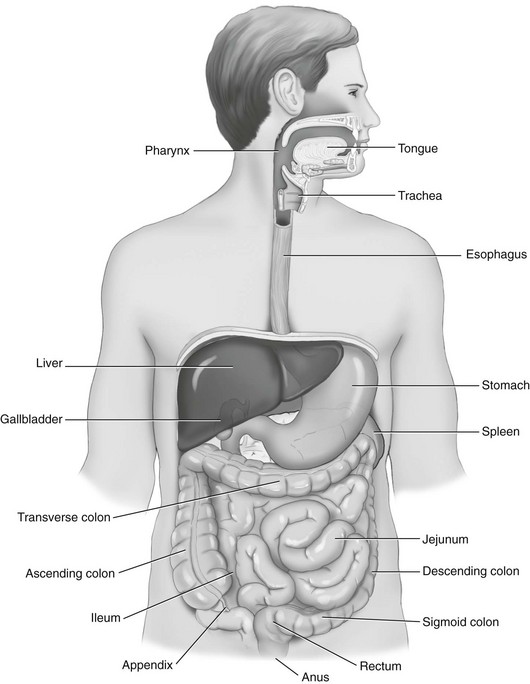
Body Structure and Function
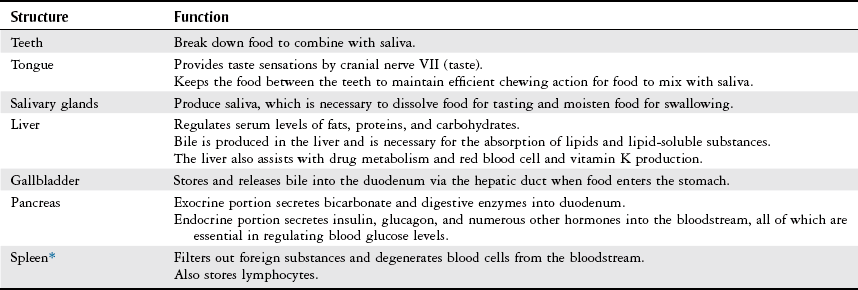
Structure
Function
Oral cavity
Entrance to the gastrointestinal system; mechanical and chemical digestion begins here
Pharynx
Involved in swallowing and mechanical movement of food to esophagus
Esophagus
Connects mouth to the stomach, transports and disperses food
Stomach (cardia, fundus, body, pylorus)
Mechanical functions: storage, mixing, and grinding of food and regulation of outflow to small intestine
Exocrine functions: secretion of hydrochloric acid, intrinsic factor, pepsinogen, and mucus necessary for digestion
Endocrine functions: secretion of hormones that trigger the release of digestive enzymes from the pancreas, liver, and gallbladder into the duodenum
Small intestine (duodenum, jejunum, ileum)
Duodenum: neutralizes acid in food transported from the stomach and mixes pancreatic and biliary secretions with food
Jejunum: absorbs nutrients, water, and electrolytes
Ileum: absorbs bile acids and intrinsic factors to be recycled in the body—necessary to prevent vitamin B12 deficiency
Large intestine (cecum; appendix; ascending, transverse, descending, and sigmoid colon; rectum; anus)
Absorbs water and electrolytes
Stores and eliminates indigestible material as feces
Clinical Evaluation
History
Physical Examination
Inspection
Auscultation
Percussion
Palpation
Diagnostic Studies
Test
Description
Carcinoembryonic antigen (CEA)
Reference value:
Adult nonsmoker <2.5 ng/ml
Adult smoker: up to 5 ng/ml
Purpose: protein used to monitor recurrence of colorectal cancer and response to antineoplastic therapy for both colorectal and breast cancer. Presence of CEA in other body fluids maybe indicative of metastasis.
Gastrin
Reference value: 0-180 pg/ml
Hormone that stimulates the release of gastric acid in stomach.
Purpose: to confirm the diagnosis of Zollinger-Ellison syndrome and monitor for recurrence of gastrinoma (gastrin-producing tumor).
Helicobacter pylori tests
Purpose: to confirm the diagnosis of H. pylori infection, which is the cause of most peptic ulcers and chronic gastritis and is a carcinogen for gastric carcinoma.
Stool sample
Reference value: negative for H. pylori antigen
Purpose: to identify presence of H. pylori antigen.
Serologic test
Reference value: immunoglobulin G negative
Purpose: to identify the presence of immunoglobulin G antibody to H. pylori in the blood.
Urea breath test
Reference value: negative
Purpose: to identify the presence of H. pylori in the stomach.
Tissue biopsy
Reference value: negative for H. pylori
Purpose: to visualize H. pylori bacteria.
A tissue biopsy is obtained during an endoscopy procedure and microscopically examined.
5-Hydroxyindoleacetic acid (5-HIAA)
Reference value: 1-9 mg/24 hr (urine sample)
Purpose: to diagnose a carcinoid tumor and provide ongoing evaluation of tumor stability.
5-HIAA is a urinary metabolite of serotonin and is produced by most carcinoid tumors found in the appendix, intestine, or lung.
Lactose tolerance test (oral lactose tolerance test)
Reference value:
Blood glucose >20 mg/dl
Purpose: to identify lactose intolerance or lactase deficiency as a cause of abdominal cramps and diarrhea, as well as to help identify the cause of malabsorption or maldigestion syndrome.
An oral dose of lactose is provided to a fasting patient, and serial blood samples are measured.
Minimal rise in blood glucose or urine lactose levels indicates lactose intolerance and/or lactase deficiency.
Occult blood (fecal occult blood test, FOBT, FOB, Guaiac smear test)
Reference value: negative
Purpose: a screening tool for early diagnosis of colon cancer.
Three stool specimens over several days are collected and examined for the presence of occult (nonvisible) blood in the feces, which can be indicative of adenocarcinoma and premalignant polyps in the colon.
Serotonin (5-hydroxytryptamine)
Reference value: 50-200 ng/ml (blood)
Purpose: to detect a carcinoid tumor.
Venous blood levels of serotonin are measured, as carcinoid tumors secrete excess amounts of serotonin.
Test
Description
Barium enema (BE)
Reference value: No lesions, deficits, or abnormalities of the colon are noted.
Purpose: to investigate and identify pathologic conditions that change the structure or function of the colon (large bowel).
The colon is emptied of feces, and contrast medium (barium) is instilled rectally. Fluoroscopic and x-ray images are then taken to identify the presence of any structural anomalies as well as polyps, tumors, and diverticula.
Barium swallow (esophagography)
Reference value: No structural or functional abnormalities are visualized.
Purpose: to identify pathologic conditions that change the structure or function of the esophagus.
The patient takes repeated swallows of barium liquid while x-ray and fluoroscopic images are taken in vertical, semivertical, and horizontal positions to examine the passage of contrast medium during swallowing and peristaltic movement of the esophagus.
Colonoscopy (lower panendoscopy)
Reference value: No abnormalities of structure or mucosal surface are visualized in the colon or terminal ileum.
Purpose: to perform routine screening of the colon for the presence of polyps or tumors and to investigate the cause of chronic diarrhea, bleeding, or other undiagnosed GI complaints.
Patients are sedated, and an endoscope is inserted into the rectum and passed through the various parts of the colon. Tissue biopsy may be performed during this procedure.
Computed tomography of the GI tract (CT scan, computed axial tomography [CAT])
Purpose: to detect intra-abdominal abscesses, tumors, infarctions, perforation, obstruction, inflammation, and diverticulitis. Metastases to the abdominal cavity can also be detected. Intravenous or oral contrast may be used during the procedure.
Esophageal function studies:
Manometry
Reference value: Lower esophageal sphincter (LES) pressure: 12-25 mm Hg.
Purpose: to evaluate esophageal motor disorders that could be causing dysphagia, for placement of intraluminal devices, and for preoperative evaluation of patients who are being considered for antireflux surgery to rule out other possible diagnoses.
Esophageal pH (acid reflux)
Reference value: pH between 4 and 4.5.
Purpose: to evaluate changes in esophageal pH levels via capsule that is implanted into esophageal mucosa 5-6 cm above the gastroesophageal junction. Monitoring takes place over 24- 48 hours.
Acid perfusion test (Bernstein test)
Reference value: Negative.
Purpose: to determine that heartburn is of esophageal rather than cardiac origin.
Hydrochloric acid is instilled into the esophagus through the endoscope or nasogastric tube. Complaints of heartburn with acid instillation confirm the esophageal origin.
Esophagogastroduodenoscopy (EGD, upper gastrointestinal endoscopy)
Reference value: No abnormal structures or functions are observed in the esophagus, stomach, or duodenum.
Purpose: to identify and biopsy tissue abnormality; to evaluate the esophagus, stomach, and duodenum to investigate the cause of upper GI bleeding, dysphagia, dyspepsia, gastric outlet obstruction, gastric ulcers, or epigastric pain.
An endoscope is passed through the mouth into the esophagus to the stomach, pylorus, and upper duodenum. Tissue biopsy can be performed during the procedure.
Gallium scan (gallium-67 imaging, total body scan)
Reference value: No structural or functional abnormalities are visualized.
Purpose: to locate malignancy, metastases, sites of inflammation, infection, and abscess. A radionuclide is injected intravenously with images taken in the following time frames:
4-6 hours later to identify infectious or inflammatory disease as well as benign or malignant tumors.
24, 48, and 72 hours later to identify and/or monitor presence of pathology.
Gastrografin study
Purpose: Similar to barium swallow, except Gastrografin (diatrizoate) is used as an imaging agent.
GI bleeding scan (GI scintigraphy, abdominal scintigraphy)
Reference value: No evidence of focal bleeding.
Purpose: to evaluate the presence, source, or both, of GI bleeding.
Radionuclide-labeled red blood cells are injected intravenously, followed by intermittent imaging studies of the abdomen and pelvis for up to 24 hours.
KUB (kidneys, ureters, bladder) x-ray
Purpose: to provide x-ray image of abdomen.
Kidneys, ureters, bladder, and small and large intestines are visualized, and resulting images can be used in assisting with diagnosis of conditions to these organs.
Paracentesis and peritoneal fluid analysis (abdominal paracentesis, peritoneal tap)
Reference value: Clear, odorless, pale yellow.
Purpose: To help delineate the cause of peritoneal effusion.
The peritoneal cavity is accessed with either a long thin needle or a trocar and stylet with the patient under local anesthesia. Peritoneal fluid is collected and examined.
Peritoneal tissue biopsy can also be performed during this procedure for cytologic studies required for the differential diagnosis of tuberculosis, fungal infection, and metastatic carcinoma.
Sigmoidoscopy (proctoscopy, anoscopy)
Reference value: No tissue abnormalities are visualized in the sigmoid colon, rectum, or anus.
Purpose: Sigmoidoscopy is used as a screening tool for the anus, rectum, and sigmoid colon for cancer and to investigate the cause of rectal bleeding or monitor inflammatory bowel disease. Proctoscopy is used to view the anus and rectum. Anoscopy is used to investigate the anus.
Upper GI (UGI) series and small bowel series (small bowel follow-through)
Reference value: No structural or functional abnormalities are found.
Purpose: to detect disorders of structure or function of the esophagus, stomach and duodenum, and jejunum and ileum (the latter two are for the small bowel series).
Imaging studies of all these areas are performed while the patient drinks a barium solution. Passage of the barium through these structures can take from 30 minutes to 6 hours.
Test
Involved Systems
Purpose
Alanine aminotransferase (ALT, serum glutamic-pyruvic transaminase [GPT], SGPT)
Reference value: 4-36 IU/L
Hepatic
To detect hepatocellular disease.
Very specific in detecting acute hepatitis from viral, toxic, or drug-induced causes.
Alkaline phosphatase (ALP, total alkaline phosphatase [T-ALP])
Reference value: 0.5-2.0 King-Armstrong units/dl
Hepatic, biliary
Nonspecific indicator of liver disease, bone disease, or hyperparathyroidism.
Sensitive test for metastatic lesions to liver.
Alkaline phosphatase isoenzymes (ALP1)
Hepatic, biliary
Used to distinguish between liver and bone pathology when total serum ALP is elevated.
There are ALP isoenzymes for both liver (ALP1) and bone (ALP2).
Alpha fetoprotein (AFP, α-fetoprotein)
Reference value: <40 ng/ml
Hepatic, biliary
A tumor marker for hepatocellular cancer in nonpregnant adults.
AFP normally exists during pregnancy; otherwise, levels are very low.
Ammonia (NH3)
Reference value: 10-80 mcg/dl
Hepatic
To evaluate or monitor liver disease, hepatoencephalopathy, and the effects of impaired portal vein circulation.
Ammonia is a by-product of protein metabolism and is converted to urea in the liver.
Amylase, serum
Reference value: 30-220 U/L
Pancreatic
To assist in diagnosis of acute pancreatitis and traumatic injury to the pancreas or as surgical complication of the pancreas.
Amylase, urine
Reference values:
6.5-48.1 U/hr (1-hr test)
5000 U/24 hr (24-hr test)
Pancreatic
To confirm diagnosis of acute pancreatitis when serum amylase levels are normal or borderline elevated.
Aspartate aminotransferase (AST, serum glutamate oxaloacetate transaminase [GOT], SGOT, transaminase)
Reference value: 0-35 U/L
Hepatic
To assist in diagnosis of suspected hepatocellular disease.
AST is highly concentrated in the liver, but it is also present in skeletal muscle, kidney, and pancreas.
Bilirubin
Reference values:
Total, 0.3-1.2 mg/dl
Direct (conjugated), 0.1-0.2 mg/dl
Indirect (unconjugated), 0.2-0.8 mg/dl
Hepatic, biliary
Used to evaluate liver function, diagnose jaundice, and monitor progression of jaundice.
Total bilirubin is the sum of direct and indirect bilirubin.
Elevation in direct (conjugated) bilirubin or indirect (unconjugated) bilirubin helps to determine cause of jaundice.
Carbohydrate antigen 19-9 (CA 19-9)
Reference value: <37 U/ml
Hepatic, pancreatic
An associated tumor marker used in the diagnosis and treatment surveillance of pancreatic and hepatobiliary cancer.
Ceruloplasmin (enzyme involved in metabolism of iron)
Reference value: 18-45 mg/dl
Hepatic
Used to help establish a diagnosis of Wilson disease.
Fecal fat (fecal lipids, quantitative fat, 72-hr stool collection, quantitative stool fat)
Reference value: 2-6 g/24 hr
Pancreatic, biliary
To identify steatorrhea (high levels of fat in the feces). This can be caused by gallstones, pancreatic duct obstruction, cystic fibrosis, and small intestine disease.
Gamma-glutamyltransferase (γ-glutamyl-transferase [GT], γ-glutamyl- transpeptidase [GGT, GGTP, GTP])
Reference values:
Male, 8-38 U/liter
Female, 5-38 U/liter
Hepatic, biliary, pancreatic
Detects liver cell dysfunction and is sensitive to cholangitis, biliary obstruction, or cholecystitis.
Hepatitis panel
Reference value: negative (no presence of antibodies)
Hepatic
Distinguishes among the three common types of viral hepatitis (HAV, HBV, HCV).
Presence of distinct antibodies or antigens for a specific virus will help confirm the diagnosis and assist with treatment planning.
Lipase
Reference value: <160 U/L
Pancreatic
Used to diagnose pancreatitis and pancreatic disease. Lipase is a digestive enzyme used to digest fatty acids.
Elevated levels indicate onset of acute pancreatitis.
5′-Nucleotidase (5′N, 5′-NT)
Reference value: 2-17 IU/L
Hepatic
An enzyme specific to the liver.
Elevated levels help to identify extrahepatic or intrahepatic diseases.
Protein electrophoresis
Reference value: albumin, 3.5-5.0 g/dl
Hepatic
To identify: presence of abnormal proteins (multiple myeloma) or absence of normal proteins (liver disease) and to detect high/low amounts of different protein groups.
Serum proteins (albumin)
Reference value: 3.5-5.0 g/dl
Hepatic
Provides general information about nutritional status, the oncotic pressure of the blood, and the losses of protein associated with liver, renal, skin, or intestinal diseases.
Sweat test (sweat chloride, cystic fibrosis sweat test, iontophoresis sweat test)
Reference value:
Sodium: <70 mEq/L
Chloride: <50 mEq/L
Pancreatic
Gold standard test used to diagnose cystic fibrosis in children, as these children have higher contents of sodium and chloride in their sweat.
Test
Purpose
Computed tomography (CT) of the liver, biliary tract, pancreas, and spleen
Used to identify the presence of tumor, abscess, cyst, sites of bleeding, or hematoma in these organs.
Contrast medium may be used with CT scan of the liver and pancreas.
Endoscopic retrograde cholangiopancreatography and pancreatic cytology (ECRP)
Used to investigate the cause of obstructive jaundice, persistent abdominal pain, or both.
Generally identifies stones in the common bile duct as well as chronic pancreatitis and duodenal cancer.
Gallbladder nuclear scanning (hepatobiliary scintigraphy, cholescintigraphy, DISIDA scanning, HIDA [heptoiminodiacetic acid] scan)
Purpose: to examine the gallbladder and the biliary ducts leading out of it. Radionuclide material is injected into the patient, absorbed by the liver, and excreted through biliary ducts; can diagnose obstructions in the ducts.
Liver biliary biopsy, percutaneous
To diagnose pathologic changes in the liver and monitor disease progression.
Liver-spleen scan
A radionuclide is injected intravenously, and imaging of the liver and spleen is performed.
Used to confirm and evaluate suspected hepatocellular disease and enlargement of the liver or spleen.
Magnetic resonance cholangiopancreatography (MRCP)
Imaging test used to visualize gallbladder, biliary ducts, and pancreatic ducts. Can identify obstructions (stones) and other pathology in the gallbladder and ducts. It is the imaging modality of choice for suspected cases of primary sclerosing cholangitis.
Ultrasound of the liver, biliary tract, pancreas and spleen
Imaging tool for examining the liver, spleen, gallbladder, and pancreas.
Cyst, abscess, hematoma, primary neoplasm, and metastatic disease can be detected in the liver as well as gallstones and pancreatic abscesses and tumors.
Laparoscopy
Magnetic Resonance Imaging
Positron Emission Tomography
Health Conditions
Esophageal Disorders
Dysphagia
Classification
Possible Etiology
Oropharyngeal
Neuromuscular: stroke, Parkinson’s disease, multiple sclerosis, myasthenia gravis, head trauma, dementia, Bell’s palsy, tumors of the central nervous system
Structural: oropharyngeal tumor, infection of pharynx or neck, thyromegaly, esophageal webs, cleft palate
Esophageal
Neuromuscular: achalasia, diffuse esophageal spasm, hypertensive lower esophageal sphincter, scleroderma
Structural: peptic stricture, esophageal rings or webs, diverticuli, tumors, foreign bodies, vascular compression, mediastinal masses, spinal osteophytes, mucosal injury from infection or gastric reflux
Esophageal Motility Disorders and Angina-like Chest Pain
Gastroesophageal Reflux Disease
Barrett’s Esophagus
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Gastrointestinal System
Only gold members can continue reading. Log In or Register to continue