Fig. 1.1
(a) A surgeon performs the PST while an assistant holds the tablet. The PIVOT® software tracks the stickers placed on the bony landmarks. (b) The software analyzes the magnitude of the reduction phase of the PST. The chart shows anterior tibial translation in the lateral compartment of 4.7 mm
It is anticipated that these emerging technologies will allow objective quantification of previously subjective clinical grades, reducing interobserver discrepancies and improving diagnostic accuracy. Nevertheless, additional tools are needed to provide diagnostic and prognostic information, especially in the absence of overt signs and symptoms, a scenario often encountered in chronic degeneration of musculoskeletal tissues. To that end, advanced imaging techniques offer the possibility of early identification of tissue damage before it is appreciable with conventional imaging modalities (i.e., plain films, MRI, CT) or arthroscopic inspection. For instance, quantitative MRI UTE-T2* mapping revealed deep cartilage tissue changes following ACL injury and reconstruction [11]. Likewise, the identification of biological markers (biomarkers) indicative of acute or chronic musculoskeletal pathology is an area of extensive investigation [12, 13]. Both approaches offer the prospect of earlier diagnosis, the enhanced prognostic power, and the ability to monitor the efficacy of therapeutic interventions. However, it should be recognized that much of this research is still in development. Validation in preclinical and clinical studies must be achieved prior to widespread adoption in the general patient population. Cost is also an important aspect to these modalities and must be considered.
1.3 Injury Prevention
Diagnostic tools that provide earlier and more accurate characterization of sports injuries offer the opportunity for the introduction of therapies that stave off further tissue damage or promote healing. But even the earliest interventions following injury are costlier and likely to yield less satisfactory results than prevention of the injury in the first place. There is an increasing appreciation for the role that fatigue and impaired movement patterns play in injury. In particular, neuromuscular fatigue can alter joint biomechanics and diminish postural control, as shown following exercise of the lower and upper extremities [14, 15]. As a result, monitoring of fatigue during competition is essential to reduce injury risk, as are training programs designed to improve neuromuscular control and improve muscular endurance. As an example, a multitude of neuromuscular and educational programs have been shown to reduce the mean incidence of ACL rupture by 50 %, but the estimated effect differs largely between studies [16]. In particular, neuromuscular training programs may be most effective in reducing injury in at-risk populations (e.g., female basketball and soccer athletes), as the efficacy of prevention programs in male athletes was shown to be limited and equivocal [17]. An improved understanding of the mechanisms of sports injuries and confirmation of the efficacy of strategies aimed at minimizing their occurrence will be essential in promoting safe participation in sport, extending from the recreational athlete to the elite sportsmen.
1.4 Individualized Treatment
When injury does occur, and conservative treatments are unable to restore adequate musculoskeletal function, surgical intervention may be indicated. Despite great progress in arthroscopic surgery in the past few decades, allowing faster recovery and improved outcomes, no surgical approach is universally successful. One possible factor contributing to surgical failures may be the standardization of surgical techniques that ignore anatomic variation between individuals. It is now accepted that surgical reconstruction that restores native anatomy yields better outcomes than nonanatomic approaches [5]. As an example, ACL reconstruction has evolved from positioning bone tunnels by a clock face technique to direct visualization (or even computer-assisted localization) of the insertion sites. These improvements in surgical techniques were predicated upon improved understanding of joint anatomy.
Likewise, the introduction of double-bundle reconstruction led to more accurate restoration of native ACL anatomy. Nevertheless, there was room for improvement; further individualization of treatment was necessary. In particular, Ferretti et al. [18] found that the ACL tibial footprint length ranged from 13.7 to 22.1 mm, demonstrating the variability in anatomy among patients. As a result, measurements of the ACL insertion sites and bony structures of the knee joint are crucial for a successful individualized ACL reconstruction. That is, double-bundle reconstruction may not always be indicated. Tibial insertion site length of <14 mm is more suitable for a single-bundle technique, while insertion sites >18 mm may be better served with a double-bundle reconstruction given that two tunnels would cover a larger percentage of the native ACL insertion site [19]. The intercondylar notch is another anatomical reference that can be used when deciding between single- and double-bundle reconstruction. A notch width of <12 mm may be too small for placement of two tunnels. Similarly, a shallow notch (<12 mm) may lead to impingement of the graft [5]. See Fig. 1.2 for an example of intraoperative measurements used in individualized ACL reconstruction as it is performed at the University of Pittsburgh.
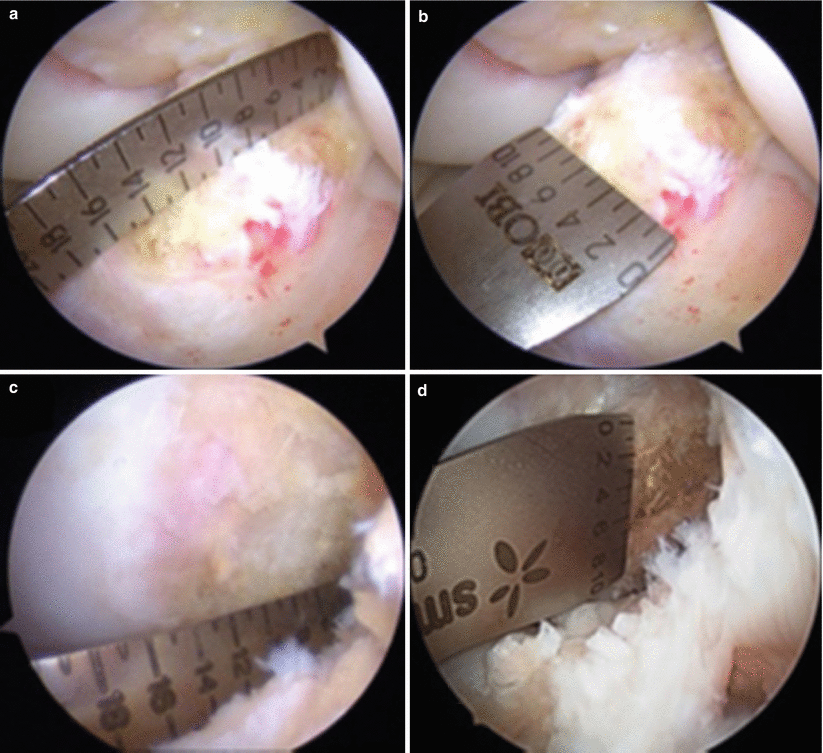

Fig. 1.2
Arthroscopic measurements of the tibial insertion site (a) length and (b) width; femoral insertion site (c) length and (d) height (From Hofbauer et al. [19] with permission)
It is important to understand that individualized anatomical ACL reconstruction is a concept rather than a technique. It can be described as four basic principles: (1) to restore the functional bundles of the ACL, (2) to restore the anatomical footprints, (3) to tension each bundle according to native tensioning pattern, and (4) to customize the surgery for each individual patient. Customizing the surgery comprises more than the decision to utilize either single- or double-bundle technique. Additional characteristics including graft choice, patients’ preferences, lifestyle, and activity level must be taken into account when planning for individualized anatomical ACL reconstruction. Beyond this, the surgeon must take into account if there is a partial or complete ACL tear and the utility of pre- and postoperative rehabilitation for each individual patient [19].
1.5 Computer-Assisted Navigation
Computer-assisted surgery (CAS) was developed with the intention to facilitate more accurate and precise results while reducing the learning curve in knee arthroplasty, trauma, and spine surgery. Efforts are now being made to incorporate computer-assisted solutions to arthroscopic surgery [20]. CAS in ACL reconstruction has now reached more than 20 years of research [21]. The main goal of navigated procedures has been to improve the graft tunnel positioning [22], as it has been shown that incorrect tunnel position is the main reason for graft failure and persistent joint instability after ACL reconstruction [23]. Several reports have shown some improvement in the accuracy of tunnel placement with computer assistance during ACL reconstruction (Fig. 1.3) [20, 25, 26]. Furthermore, CAS may facilitate learning for junior surgeons and help experienced surgeons in complicated cases [27]. It also has been a valuable tool in research due to its precision in intraoperative evaluation of joint anatomy and laxity. For these reasons, it is probable that CAS will play an increasing role in all surgical fields, including arthroscopy for sports injuries.
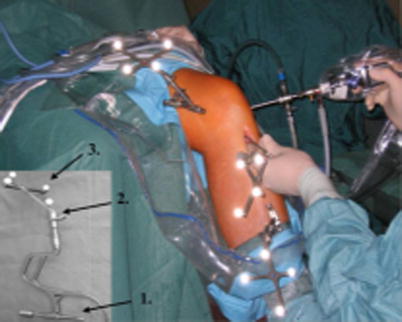

Fig. 1.3
Photograph showing arthroscopy of the left knee with computer assistance. K-wires (arrow 1) with rigid bodies (arrow 2) and reflective markers (arrow 3) are placed at the distal femur and proximal tibia to provide a connection to the OrthoPilot® navigation system (From Hofbauer et al. [24] with permission)
1.6 Biologics in Sports Medicine
Now in the third decade after the seminal publication of Langer and Vacanti [28] describing tissue engineering – the application of cells, scaffolds, and bioactive agents to replace or repair damaged tissue – there is rapidly expanding interest in using biologics to enhance healing of sports injuries [29]. While ex vivo engineering of whole organs derived from an autologous cell source may provide a novel, and desperately needed, alternative for organ transplantation, successful application of tissue engineering in sports medicine will likely require recapitulation of native tissue structure and function in focal defects (e.g., osteochondral lesions) or gap defects (e.g., nonunion fractures or tendon ruptures). Several technologies have shown promise clinically, including autologous chondrocyte implantation (ACI) [30] for focal cartilage lesions and the administration of bone morphogenetic protein-2 (BMP-2) for nonunion fractures and spinal fusion [31]. However, these early-generation biologics are not without limitations. In particular, first-generation ACI, which utilized periosteal membranes in conjunction with ex vivo expanded autologous chondrocytes, was associated with hypertrophy [32]. Additionally, the preponderance of fibrocartilage formed in the repair site, the expense of cell expansion in accordance with good manufacturing practice (GMP), and the uncertain clinical superiority over microfracture have prevented broad adoption of this biological approach. Likewise, the broader implementation of BMP-2 to improve bone healing has been slowed by questions of cost-effectiveness [31] and potential complications coupled with financial conflicts of interest that mired early reports on its efficacy [33]. While newer generations of these products may obviate several of these potential limitations, economics will continue to influence clinical care, necessitating the use of biologics in a one-step procedure. The biologics will likely be procured from the patient’s own tissues, available as off-the-shelf, time-efficient products, or some combination thereof [34] (Fig. 1.4).
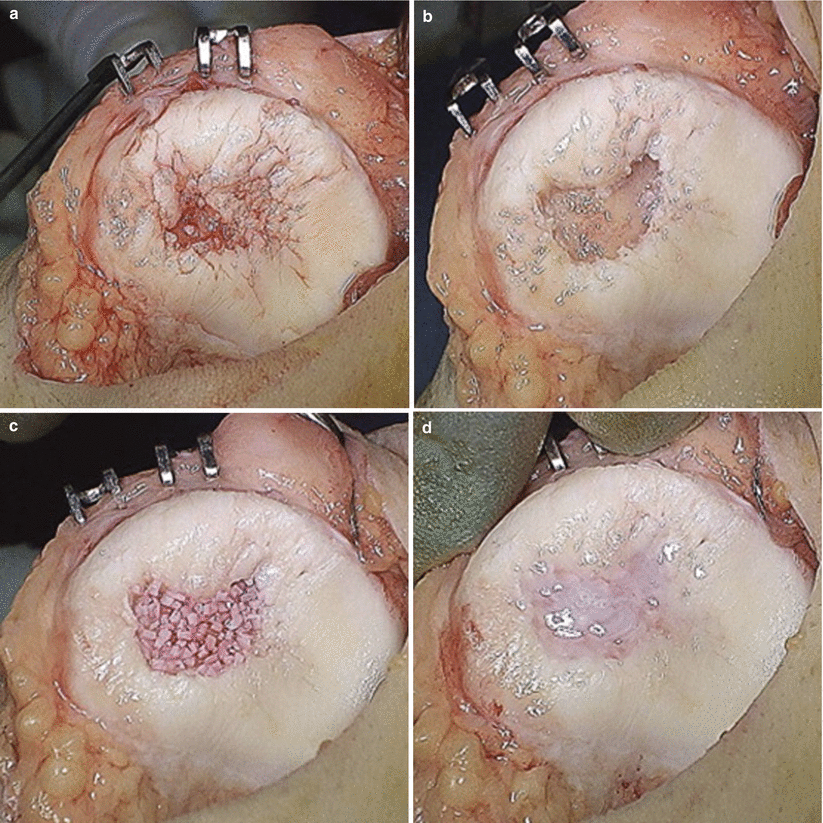

Fig. 1.4
Single-stage cartilage restoration technique utilizing particulated juvenile articular cartilage pieces placed in the chondral defect of the patella. The patella chondral defect (a) is prepared for allograft placement (b). Juvenile articular cartilage pieces are localized to the defect by fibrin glue (c). Additional fibrin glue seals the allograft in the defect (d). Similar single-step biological approaches are being explored in an effort to improve articular cartilage regeneration (Reproduced with permission from Tompkins et al. [35])
Equally important to the success of biologics in promoting healing is the growing understanding of the role of musculoskeletal loading in tissue remodeling. Recent studies on postoperative rehabilitation of musculoskeletal tissues, such as the rotator cuff [36, 37] and flexor [38] and Achilles tendons [39], point to the need for controlled mobilization [40]. However, the relationship between physical therapy and biologics remains largely unknown [41]. Interestingly, several preclinical models suggest a synergistic effect between biologics and mechanical loading. Hodde et al. [42] demonstrated that early motion of an Achilles defect augmented with a small intestine submucosa scaffold produced superior tissue remodeling when compared against immobilized joints. Likewise, Virchenko and Aspenberg [43] found that platelet-rich plasma interacted synergistically with mechanical loading to improve structural properties of a healing Achilles tendon in a rat model, but this effect was most pronounced when loading began after the inflammatory phase of healing (~5 days). In a similar study, Ambrosio et al. [44] showed that the engraftment of mesenchymal stem cells in damaged muscle tissue was enhanced by functional muscle stimulation. Clearly, future investigations to elucidate the complex mechanical and biochemical microenvironment of healing musculoskeletal tissues, coupled with a growing understanding of the mechanisms by which biologics exert favorable effects, will be essential to fulfill the promise of biologics in sports medicine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







