Chapter 92 Flavonoids—Quercetin, Citrus Flavonoids, and Hydroxyethylrutosides
 Introduction
Introduction
Flavonoids, a group of plant pigments, are largely responsible for the colors of many fruits and flowers. Recent research suggests that flavonoids may be useful in the treatment and prevention of many health conditions. Many of the medicinal actions of foods, juices, herbs, and bee pollen are now known to be directly related to their flavonoid content. More than 4000 flavonoid compounds have been characterized and classified according to their chemical structure. Flavonoids are a bit of an enigma to scientists, because they are quite reactive compounds. They can enter into almost any type of reaction known to organic chemistry, such as oxidation-reduction reactions, carbonyl reaction, acid-base reactions, free-radical reaction, hydrophobic interactions, tautomery, and isomerizations.1 As such, characterization of the many diverse physiologic properties of flavonoids is a considerable challenge to biochemists and researchers alike. This chapter discusses a few representatives of this class of useful clinical agents (quercetin, citrus bioflavonoids, and hydroxyethylrutosides).
Another beneficial group of plant flavonoids are the proanthocyanidins (also referred to as procyanidins). Collectively, mixtures of proanthocyanidin dimers, trimers, tetramers, and larger molecules are referred to as procyanidolic oligomers (PCOs). Chapter 117 discusses PCOs.
 Chemical Descriptions
Chemical Descriptions
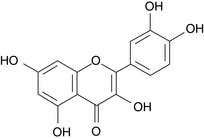
Quercetin
Quercetin (Figure 92-1) is a flavonoid that serves as the aglycone for many other flavonoids, including the citrus flavonoids rutin, quercitrin, and hesperidin. These derivatives differ from quercetin in that they have sugar molecules attached to the quercetin backbone. Quercetin is consistently the most active of the flavonoids in experimental studies, and many medicinal plants owe much of their activity to their high quercetin content.
 Pharmacology
Pharmacology
As a class of compounds, flavonoids have been referred to as “nature’s biological response modifiers” because of their ability to modify the body’s reaction to other compounds, such as allergens, viruses, and carcinogens, as evidenced by their anti-inflammatory, antiallergic, antiviral, and anticarcinogenic properties.2 In addition, flavonoids act as powerful antioxidants, providing remarkable protection against oxidative and free radical damage;3 they also have the ability to traverse the blood–brain barrier, thus exerting a neuroprotective effect.4 Flavonoids are known to form strong ligand complexes with heavy metal ions and may prove to be a good agent of heavy metal detoxification.1
The practical aspect of this antioxidant activity was highlighted by the results of a study in 805 men designed to determine the effect of dietary flavonoids on protecting against heart disease. The study demonstrated an inverse correlation between flavonoid intake and death from a heart attack.5 This effect was probably a result of the potent antioxidant effects of the flavonoids preventing, similar to vitamins C and E, the formation of oxidized cholesterol. However, the antioxidant activity of flavonoids is generally more potent and effective against a broader range of oxidants than traditional antioxidant nutrients like vitamins C and E, selenium, and zinc.6,7
Quercetin
Antiinflammatory Effects
Quercetin has demonstrated significant anti-inflammatory activity due to direct inhibition of several of the initial processes of inflammation via interaction with calcium channels or calmodulin (the intracellular calcium-binding protein), or both. It works through other mechanisms as well, such as inhibiting mast cell and basophil degranulation, neutrophil and monocyte lysosomal secretion, prostaglandin (most notably, leukotriene) formation, lipid peroxidation, and the resultant cascade of effects that are often a result of these processes. For example, it inhibits both the manufacture and release of histamine and other allergic/inflammatory mediators. In addition, it exerts potent antioxidant activity and vitamin C–sparing action.6–9
Effect on Histamine Release
The release of histamine and other inflammatory mediators from mast cells and basophils is involved in the pathogenesis of acute allergic and inflammatory responses. Mast cells are widely distributed throughout the human body but are found in higher concentrations in the blood vessels of the subepithelial connective tissue of the respiratory tract, conjunctiva, gastrointestinal tract, and skin. Mast cell and basophil degranulation is an active process that requires calcium influx. Quercetin and many other flavonoids have been shown to be potent inhibitors of mast cell, neutrophil, and basophil degranulation. A generally accepted hypothesis for this action is that quercetin inhibits receptor-mediated calcium influx, thereby inhibiting the primary signal for degranulation. However, quercetin is also active under conditions in which the calcium channel mechanism is not operative, indicating that other mechanisms are responsible as well.6–9
Membrane Stabilization, Antioxidant Activity, and Hyaluronidase Inhibition
Quercetin inhibits many of the inflammatory processes attributed to activated neutrophils. This effect is probably due to its membrane-stabilizing action, potent antioxidant effect (which prevents the production of free radicals and inflammatory leukotrienes), inhibition of the enzyme hyaluronidase (thus preventing the breakdown of the collagen matrix of connective tissue and ground substance), and finally inhibition of the proinflammatory cytokines. Quercetin’s membrane-stabilizing effect could also account for its action in preventing mast cell and basophil degranulation. This effect also inhibits inflammation by decreasing neutrophil lysosomal enzyme secretion.10 Neutrophils and monocytes contain lysosomes that, on secretion of their contents, contribute greatly to the inflammatory process.
Effects on Eicosanoid Metabolism
Excessive leukotriene formation has been linked to asthma, psoriasis, atopic dermatitis, gout, ulcerative colitis, and possibly cancer. Quercetin has been shown to inhibit many steps in eicosanoid metabolism. Its inhibition of phospholipase A2 and lipoxygenase enzymes is probably its most significant action (see Chapter 149 for diagram). The net result is a significant reduction in the formation of leukotrienes. The leukotrienes C4, D4, and E4 (composing the slow-reacting substances of anaphylaxis) are derived from arachidonic acid and are 1000 times as potent as histamine in promoting inflammation. Leukotrienes promote inflammation by causing vasoconstriction (thereby increasing vascular permeability) and bronchoconstriction (thus inducing asthma) and by promoting white blood cell chemotaxis and aggregation. The reduction of leukotriene formation by quercetin has significant anti-inflammatory effects.
Inhibition of Aldose Reductase
Quercetin is a strong inhibitor of aldose reductase, the enzyme responsible for the conversion of blood glucose to sorbitol. This compound is strongly implicated in the development of diabetic complications such as diabetic cataracts, neuropathy, and retinopathy.11 The mechanism by which sorbitol is involved in the development of diabetic complications is best understood by considering its involvement in cataract formation. Although the lens does not have any blood vessels, it is an actively metabolizing tissue that continuously grows throughout life. Elevated blood sugar levels result in shunting of glucose to the sorbitol pathway.
Quercitrin, which is hydrolyzed by gut bacteria to yield quercetin and a sugar moiety, was shown to significantly decrease the accumulation of sorbitol in the lens of diabetic animals, effectively delaying the onset of cataracts.12 In addition to its effect on aldose reductase, quercetin is also of value in diabetes for its ability to enhance insulin secretion and protect the pancreatic β cells from the damaging effects of free radicals, and for its inhibition of platelet aggregation.6,7
Antiviral Activity
Flavonoids as a group possess significant antiviral activity, with quercetin having the greatest antiviral activity against herpes virus type I, parainfluenzae 3, polio virus type I, and respiratory syncytial virus.13 Quercetin was shown, in vitro, to inhibit both viral replication and infectivity. In vivo studies in animals also showed quercetin to inhibit viral infection.14 This would suggest that quercetin might be of some benefit in viral infections, including the common cold.
Anticancer Properties
Many flavonoids have also been shown to inhibit tumor formation, but again quercetin has consistently been the most effective. In experimental models, quercetin demonstrated significant antitumor activity against a wide range of cancers, including squamous cell carcinoma, leukemia, and cancers of the breast, ovaries, colon, rectum, and brain. The cancer preventive effects of quercetin have been attributed to various mechanisms, including antioxidative activity, inhibition of enzymes that activate carcinogens, modification of signal transduction pathways, and interactions with receptors and other proteins, such as the androgen receptor involved in the development and progression of prostate cancer.15–17
Clinically, quercetin holds great promise in reversing multidrug resistance (MDR) in cancer cells. Transporter-mediated active efflux of cytotoxic agents is one of the best characterized mechanisms by which cancer cells develop MDR. Quercetin demonstrated multitargeted effects in reversing MDR.17
Citrus Flavonoids
Collagen Matrix Support
They reinforce the natural cross-linking of collagen that forms the so-called collagen matrix of connective tissue and protect against free radical damage with their potent antioxidant and free radical scavenging action. They also inhibit enzymatic cleavage of collagen by enzymes secreted by leukocytes during inflammation and microbes during infection. Like quercetin, citrus flavonoids also prevent the release and synthesis of compounds that promote inflammation and allergies, such as histamine, serine proteases, prostaglandins, and leukotrienes.8,9 It is believed that the citrus bioflavonoid hesperidin possesses antihistaminic activity through its metabolite heparitin, a weak inhibitor of cyclooxygenase-2 enzymes. This metabolite is produced as a product of intestinal bacteria, which underscores the need for a balanced intestinal flora to gain the antihistamine benefits of citrus bioflavonoids.18
Bone Metabolism Effects
Histomorphometric research on ovariectomized mice showed that marked decreases in trabecular bone volume and trabecular thickness of the femoral distal metaphysis were significantly prevented by hesperidin. Additionally, calcium, phosphorus, and zinc concentrations in the femur were significantly higher in the hesperidin-fed group, whereas serum and hepatic lipids were lower in mice that consumed hesperidin-containing diets.19
Pharmacokinetics
Before discussing clinical applications of quercetin, it is important to address absorption and metabolism. Previous pharmacokinetic studies in animals and humans indicated that little quercetin is absorbed intact, with the majority of the oral dose (53%) being excreted in the feces.20,21 One of the main problems in studying the absorption of quercetin and other flavonoids is their degradation by microorganisms in the colon. To sidestep this issue, one study examined the absorption of quercetin in healthy ileostomy patients with complete small intestines.22 The study examined the absorption of quercetin from fried onions (a rich source of quercetin glycosides), rutin, or 100 mg of pure quercetin. Absorption was defined as oral intake minus ileostomy excretion and corrected for degradation within the ileostomy bag. Absorption results were as follows: 52% from onions, 17% from quercetin rutinoside, and 24% for pure quercetin. These results indicate that humans do absorb appreciable amounts of quercetin and that absorption (but not necessarily pharmacologic activity) may be enhanced when quercetin is bound to glucose. In other words, citrus bioflavonoid preparations or HERs, or both, may prove to be more effective clinically.
Subsequent studies shed additional light on the absorption and metabolism of quercetin. In a study of 35 healthy volunteers, the volunteers were randomly assigned to take 50, 100, or 150 mg/day (groups Q50, Q100, and Q150, respectively) quercetin for 2 weeks. Fasting blood samples were collected at the beginning and end of the supplementation period. Compared with baseline, quercetin supplementation significantly increased plasma concentrations of quercetin by 178% (Q50), 359% (Q100), and 570% (Q150). Pharmacokinetics of quercetin were investigated in a subgroup of 15 volunteers. The areas under the plasma concentration–time curves ranged from 76.1 to 305.8 µmol/min/L(−1) (50- and 150-mg doses, respectively). Median maximum plasma concentrations of quercetin (431 nmol/L) were observed 360 minutes after intake of 150 mg quercetin.23
In a much larger study, 1002 subjects were randomized to one of three groups: Q-500 (500 mg/day), Q-1000 (1000 mg/day), or placebo. Quercetin supplementation over 12 weeks caused a significant increase in overnight-fasted plasma quercetin, with a net increase of 332 and 516 mcg/L for Q-500 and Q-1000 compared with 53.6 mcg/L for placebo. However, the increase in plasma quercetin was highly variable within each quercetin supplementation group.24
Once absorbed, much of absorbed quercetin is conjugated to glucuronic acid in the liver by phase II enzymes. It is thought that β-glucuronidase released from neutrophils at inflammatory sites may be able to deconjugate and thus activate quercetin glucuronides. This effect was demonstrated in vitro.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree