Chapter 91 Fish Oils (Omega-3 Fatty Acids, Docosahexaenoic Acid, Eicosapentaenoic Acid, Dietary Fish, and Fish Oils)
 Introduction
Introduction
Initial scientific evidence of fish oil’s benefits first appeared in the late 1970s, when it was claimed that fish oil consumption among Greenland Eskimos and other fish-eating populations might convey a lifetime protective effect against coronary heart disease (CHD).1–3 A large study in the United States, the Diet and Reinfarction Trial (DART),4,5 and two studies in Europe, the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI)-Prevenzione Trial and the Lyon Diet Heart Study,6–8 supported the observational evidence from these indigenous populations, and demonstrated that by increasing dietary ω-3 fatty acid intake, a significant decrease in ischemic heart disease occurred. More recently, the Japan Eicosapentaenoic Acid (EPA) Lipid Intervention Study (JELIS) trial randomized nearly 20,000 individuals to either a statin or a statin plus EPA, and found a 19% reduction in major coronary events in patients using supplemental EPA, as well as a significant reduction in stroke.9,10 In 2010, the Heart and Soul study documented a significantly lower risk of overall mortality in individuals with higher blood levels of EPA and docosahexaenoic acid (DHA).11 This latter study is particularly important, because it suggested that we have a biomarker for risk assessment, one that may avoid some of the biases inherent in food frequency questionnaires, variability in fish oil products, etc.
These studies and many others have had a profound effect on public health policymakers’ perceptions of the importance of fish and fish oil to human health. For example, on February 8, 2002, the U.S. Food and Drug Administration (FDA) announced that it would permit the claim “consumption of omega-3 fatty acids may reduce the risk of CHD,” to appear on labels of ω-3 fatty acid supplements containing a dose level of DHA and EPA of up to 2000 mg/day. The FDA’s decision to permit such a claim is significant and timely, in that by 2000, one million Americans were reported to have heart attacks annually. On the basis of these and several other studies, it is now estimated that this one change in diet could save at least 150,000 people annually from fatal heart attacks.12–17 Of course, increased daily intake of 5 to 7 servings of fruits, vegetables, and nuts rich in antioxidants will also reduce the incidence of heart attacks.
In 2002, the American Heart Association (AHA) released a position paper advocating the consumption of oily fish twice per week among those without heart disease, and at least 1 g/day of EPA/DHA among those with established coronary artery disease (CAD).18 This marks the first time the AHA recommended a nutritional supplement for CAD prevention.
 Description
Description
Marine life is generally rich in two ω-3 fatty acids (also referred to as n-3 fatty acids), EPA and DHA, which enter the food chain through phytoplankton. These long-chained and highly polyunsaturated fatty acids (PUFAs) contain, respectively, 20 and 22 carbons and 5 and 6 double bonds, also referred to as 20:5n-3 PUFA and 22:6n-3 PUFA. The 20:5n-3 PUFAs and 22:6n-3 PUFAs are abundant in shellfish, sea mammals, and fish, hence, the reason Greenland Eskimos’ diet is rich in EPA and DHA. By comparison, EPA and DHA levels are low or absent in domesticated land animals, in part because mammals lack the enzymes needed to insert a double bond in the n-6 or n-3 position. Thus, linoleic acid and α-linolenic acid (ALA), rich in a number of plant oils, are essential fatty acids, and, slowly with significant genetic variation, enzymatically converted to EPA and DHA. Fish oil from herring, cod liver, wild salmon, mackerel, and sardines is particularly rich in ω-3 fatty acids containing EPA and DHA. Table 91-119 lists the relative concentration of fatty acids in various common fish species.
TABLE 91-1 Relative Concentration of Fatty Acids in Fish Oils and Mercury (Hg) in the Respective Fish
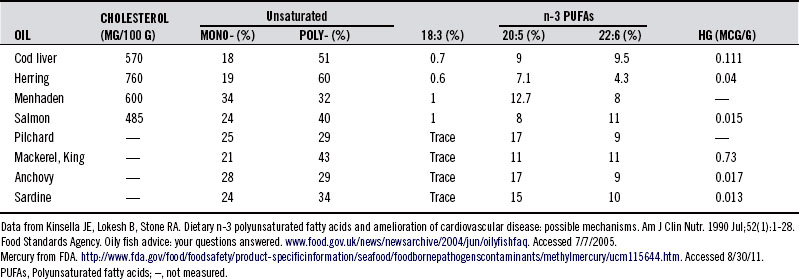
The habitat in which fish grow has a major impact on their fatty acid composition.20 In the wild, fish consume food sources that contain high levels of ALA (18:3n-3). Fish raised in “fish farms” deserve mention. It has been reported that salmon raised in fish farms receive a steady diet of synthetic pigment to give them a rich pink hue; otherwise, they would be unappetizing given their rather morbid, pale gray appearance. In the natural ocean environment, phytoplankton, which is rich in EPA and DHA, form the basis of the food chain for salmon and contribute to its pink color. Commercial fish foods contain less DHA and EPA, and therefore, lower concentrations of ω-3 fatty acids. Studies showed that wild fish have higher concentrations of ω-3 fatty acids than pond-reared and/or cultured fish grown in fish farms and fed commercial feedstuffs that are devoid of EPA and DHA.21 Additionally, although wild fish are also increasingly contaminated, farmed fish often contain higher levels of toxins, such as organochlorines, brominated flame retardants, and methylmercury, particularly when they are also given fish meal as a food source.22,23
For individuals who do not or cannot consume seafood, foods such as tofu, canola oil, black currant oil, flaxseed oil (the best non-fish source), nuts, and soybeans are important sources of ALA (18:3n-3). However, soy-derived oils and foods and most nuts contain large amounts of ω-6 fatty acids that can reduce some of the therapeutic benefits of the ω-3 fatty acids, so finding a prudent balance between ω-3 and ω-6 fatty acids is suggested. Additionally, one analysis indicated that a dramatic 1000-fold increase in the consumption of soybean oil over the last century increased ALA intake, but it was offset by the increase in lineoleic acid, and therefore likely to depress tissue levels of EPA and DHA.24 A recent review found that the fatty acids found in fish oil, particularly DHA, are substantially lower in tissues of vegetarians and vegans, including plasma levels, breast milk, and blood cells.25 Fortunately, organically derived supplemental DHA and EPA from algae is now available for those wishing to avoid fish consumption.26
Dietary Requirement
The first suspected case of ω-3 fatty acid deficiency in humans was not reported until 1988, and the optimal intake of ω-3 fatty acids, both plant and marine-based, is still not well-established.27 No Adequate Intakes (AIs) or Dietary Reference Intakes (DRIs) have been set so far by the Institute of Medicine for EPA and DHA or the long-chain ω-6 arachidonic acid (AA), although it has established AIs for the short-chain ω-3 (ALA of 1.1 g for women and 1.6 g for men). There are several factors that complicate the establishment of clear dietary requirements, and several lines of evidence that offer some guidance. Evolutionary intakes of ω-3 fatty acids ALA and EPA+DHA estimated by Kuipers et al27a suggest a probable value of 9g/day (range 8.7 to 9.3g) of ALA and 3.9g/day (range 0.8 to 9.7g) of EPA+DHA per 2000 Kcal/day.
One of the factors involved is the proportional intake of ω-6 fatty acids to ω-3 fatty acids. It appears that the ratio of these two types of fatty acids, as well as their total intake, is important in determining health outcomes, and a change in both variables has occurred in Western diets over the last 150 years. Currently, the ratio of n-6:n-3 fatty acids in a typical Western diet is 15 to 20:1, whereas in wild animals, as well as humans throughout most of evolutionary history, it has been approximately 1:1.28 This dramatically shifted ratio has skewed the metabolic products (prostaglandins, thromboxanes, leukotrienes, hydroxy fatty acids, and lipoxins) derived from AA (vs n-3 acids) to favor a more inflammatory, prothrombotic, proaggregratory, provasoconstrictive, and proproliferative physiologic state. The n-6:n-3 ratio was found to be important not only to the pathogenesis of cardiovascular diseases, but also in cancer, inflammatory, and autoimmune diseases, as well as in brain function.29
One example that highlights the importance of the ratio between oils was demonstrated in a study of over 400 healthy men and 450 healthy women. Researchers found that only at high n-6 intakes were DHA and EPA intakes inversely associated with some markers of inflammation, and a combination of both was associated with the lowest inflammation.30 Similarly, the intake of n-3 fatty acids in women with a high intake of n-6 fatty acids has an inverse relationship with the risk for breast cancer.31 This was confirmed in a very large prospective cohort study of Chinese women (Shanghai Women’s Health Study), in which the relative intake was more important to the risk of breast cancer than total intake.32 In other words, the consequence of low n-3 intake is particularly important given a background of high n-6 intake.
Another contributing factor may be variations in the activity of enzymes involved in eicosanoid synthesis. For example, there is some conversion from ALA to EPA and DHA, but this conversion is fairly limited, and has significant interindividual variation.33 This conversion from ALA is estimated to be approximately 5% for EPA and less than 0.5% for DHA, although the desaturase enzymes involved are influenced by smoking, gender, age, trans fat, and n-6 fatty acid intake. For example, a recent study found that despite large differences in intake of n-3 fatty acids between fish eaters and non-fish eaters, the plasma levels were much closer than expected, likely due to increased conversion.34 Also of importance is the individual variability of the activity of enzymes involved in mediating the physiologic effects of fish oil. For example, variants in the 5-lipoxygenase (ALOX5) gene appear to modify the effects of fish oil on cardiovascular risk, as do polymorphisms in peroxisome proliferator activated receptor-α (PPAR-α).35–37
Despite these limitations, most recommendations suggest a desired DHA and EPA total intake of approximately 0.5 to 1 g/day as preventative therapy, with higher doses indicated for treating specific conditions. A ratio of 2:1 to 1:2 EPA/DHA is often advocated, depending on therapeutic goals. For example, the Technical Committee on Dietary Lipids of the International Life Sciences Institute (North America) suggests an intake between 250 and 500 mg DHA+EPA per day for CAD prevention. Currently, even a 500 mg target would require at least a tripling of current intake in most Western countries.38
Because the concentration of EPA and DHA in fish can vary depending on its growth environment, fish oil supplements can offer a viable and reliable source of ω-3 fatty acids standardized around the content of EPA and DHA (see Table 91-1). Most fish oil capsules contain between 300 and 500 mg EPA+DHA fatty acids per gram, while some are concentrated to contain as much as 700 mg EPA+DHA per gram. Patients using fish oil at the therapeutic levels discussed herein may require between 15 and 30 capsules daily to derive the described benefits, depending on their DHA+EPA content. At 9 calories/g of oil, this can represent a significant increase in caloric intake and may necessitate an increase in energy expenditure to avoid weight gain. We recommend using fish oil supplements which are highly concentrated to decrease unnecessary calorie intake.
EPA- and DHA-rich fish oil should be kept in capsules (such as soft gelatin with minimal plasticizer content39; see later discussion on “Oxidation”) capable of providing an oxygen barrier. Otherwise, toxic lipid peroxides (e.g., malondialdehyde) and anisidine isomers may form. Encapsulated liquid sources generally do not have this protection unless they contain antioxidants. Encapsulated products typically contain fish oil, not fish liver oil. This distinction is important because fish liver oil contains the fat-soluble vitamins which, if taken in excessive amounts, can cause vitamin A toxicity, although some brands are reducing the vitamin A content during processing.
Explaining Inconsistent Results of Fish Oil Supplementation Trials
A number of articles in the fish oil literature have reported equivocal or contradictory results. Many of these differences in outcomes can be explained by the use of olive oil as a placebo or the lack of control for saturated or ω-6 fatty acid intake, as well as an insufficient dose of n-3 fatty acids. For example, in the recent Alpha Omega trial, nearly 5000 patients with a previous myocardial infarction were only given 400 mg DHA/EPA, a dose less than one half used in previous trials.40 Not surprisingly, this low dose did not improve the rate of major cardiovascular events. It also raised plasma levels to a much lower degree than in previous trials that used a higher dosage.
Olive oil cannot be considered an inert placebo in trials that investigate effects on platelet function or CHD risk. Several studies showed that olive oil supplementation has similar inhibitory effects on various aspects of platelet function, including decreased platelet aggregation and thromboxane A2 (TXA2) release, increased platelet membrane oleic acid content, and decreased platelet membrane AA content.41,42 Further, an excess of oleic acid impairs incorporation of AA into platelet phospholipids.
An additional consideration that is often ignored is the background intake of the enrolled study population. For example, an increase in n-3 intake in Western populations showed a reduction in the risk of sudden death, a benefit not consistently shown in Japanese populations, where the background intake is much higher.43
The lack of a relevant biomarker, such as the ω-3 index (discussed in the following section), as well as the lack of consideration of an individual’s genetic response, are also variables that certainly contribute to the appearance of ineffectiveness. For example, studies that use food frequency questionnaires often do not accurately gauge n-3 intake. Additionally, they do not take into account differences in fatty acid metabolism that are vital to determining more functional measures. For example, a recent analysis of over 700 individuals found that supplements and the intake of EPA/DHA rich food explained less than one half of the variability in red blood cell content, which may be due to the individual intake of linoleic and/or arachidonic acid, since they all compete for incorporation in the cell membranes.44
Another possible complicating factor is the squalene found in olive oil and some deepwater fishes, but not in other vegetable oils.45 Other problems include the differing ratios of fatty acids, the position of the fatty acids on the glycerol backbone, and the susceptibility to peroxidation of the various fish oil preparations.46
Also, overprocessing of fish oil supplements can result in a loss of its active constituents and, hence, an attendant decrease in its efficacy. This was demonstrated in at least two studies that looked at such variables as how processing affects levels of triglycerides, cholesterol, lipoprotein(a), atherogenic index, and fibrinogen after fish oil supplementation.47,48
 Pharmacology
Pharmacology
The effects of supplemental and dietary unsaturated fatty acids appear to be mediated through changes in serum lipids, altered ratios of prostaglandins, decreased platelet aggregation, and modification of cell membrane activity.46 Additionally, n-3 fatty acids have been shown to modify gene expression, and to directly reduce inflammation by binding to n-3 specific receptors. Omega-3 fatty acids (EPA, DHA and docosapentaenoic acid [DPA]) are also the metabolic precursors to the resolvins, lipoxins, and protectins now shown to be essential to the resolution of inflammation.
In 2010, researchers published a breakthrough study in the journal Cell, which for the first time identified a new g-protein coupled receptor, GPR120, which “functions as an n-3 FA receptor/sensor in proinflammatory macrophages and mature adipocytes.”49 Through this receptor, DHA and EPA mediated robust antiinflammatory signaling, inhibiting both the toll-like receptor and tumor necrosis factor-α (TNF-α) pathways. This finding was quite important, in part because insulin resistance is modified by these pathways, and also because these same researchers were able to document a direct insulin-sensitizing effect by EPA and DHA mediated via this receptor. This provides a direct and specific mechanism for improving insulin sensitivity.
In recent years, fish oils have also been shown to be the precursors for the modulators of the resolution phase of inflammation.50 Resolvins, derived from EPA, and protectins, derived from DHA, have been shown to be crucial to both the resolution of inflammation as well as the initiation of tissue repair in multiple body systems. This modulation of inflammation by fish oil has also dramatically changed the way inflammation is viewed. It is now understood to be a carefully orchestrated active process, dependent upon adequate intake of eicosanoid precursors, rather than a passive process.
Fish oils also appear to reduce triglycerides and decrease the production of the prothrombotic substance TXA2 by occupying TXA2 receptors and enhancing the production of the platelet antiaggregatory substance prostacyclin. These actions play an important role in inflammation, atherogenesis, thrombosis, and CHD. Fish meals that provide an average of 3.6 g/day of ω-3 fatty acids reduce platelet aggregation and platelet TXB2 responses.51 Significant decreases in platelet sensitivity to collagen, serum TXB2 levels, and urinary TXB2 metabolites were observed after ω-3 fatty acid treatment.52 EPA supplementation was shown to decrease synthesis of the ω-6 platelet agonist TXA2 coincident with formation of the inactive ω-3 form, TXA3.53,54 A longitudinal study showed a pronounced action of dietary ω-3 fatty acids to diminish platelet formation and endothelial deposition, which could lead to thrombosis.55
Through the vasodilatory effects of prostaglandin I3 (PGI3), fish oils may improve peripheral circulation, thereby assisting very low-density lipoprotein (VLDL) cholesterol removal. This may be accomplished by altering membrane fluidity in a specific manner, thus affecting the activity of membrane-bound enzymes and resulting in changes in receptor activity, specificity, and signal transduction. The incorporation of fish oil into membranes was shown to have direct clinical consequences. For example, in a case–control study of over 300 patients, a higher red blood cell membrane ω-3 fatty acid content was associated with a 70% risk reduction in cardiac arrest.56 Evidence also suggested that fish oils decreased hepatic synthesis of fatty acids and triglycerides and reduced secretion of VLDL cholesterol while displacing AA from tissue phospholipids. This results in ω-3 fatty acid levels that inhibit thromboxane synthesis.
Researchers noted that fish oil effects are selective. EPA and DHA not only displace AA from phospholipid pools and inhibit cyclooxygenase, but EPA also becomes a preferred substrate for cyclooxygenase when peroxide tone is high. This results in decreased production of the vasoactive and aggregatory prostacyclin (PGI2) and increased production of PGI3, which has more potent antiaggregatory effects. According to some researchers, increased bleeding time is due to either less TXA2 or higher prostacyclin I3 levels,53 although others contend that EPA conversion to PGI3 is the primary cause.57 Many researchers believe this change is one of the primary factors that decreases the risk of atherosclerosis and thrombosis.58–64 These findings may explain some of the epidemiologic evidence of decreased CAD and prolonged bleeding time seen in some Eskimos and in those Japanese found eating a diet rich in fish.65 Recent analyses also indicated that increases in bleeding time might have been exaggerated, with all current data pointing to minimal, if no affect.
Fish oils rich in EPA and DHA were also found to suppress production of inflammatory mediators found in patients with RA and psoriasis. The antiinflammatory effect of the ω-3 fatty acids is probably due to reduced production of leukotrienes, interleukin-2 (IL-2), and TNF-α, all principal mediators of inflammation. One study found that the ability of fish oil to decrease TNF-α production was influenced by an inherent TNF-α production and by polymorphisms in the TNF-α and lymphotoxin α genes (also known as TNF-β).66
• Decrease production of prostaglandin E2 (PGE2) metabolites
• Decrease production of TXA2 (an active vasoconstrictor and platelet aggregator)
• Increase prostacyclin PGI3 (an active vasodilator and inhibitor of platelet aggregation)
• Increase production of leukotriene B5 (a weak inducer of inflammation; weak chemotactic agent)
• Decrease production of leukotriene B4 (a weak inducer of inflammation; inducer of leukocyte adherence and chemotaxis)
Fish oil, particularly DHA, was shown to modify mitochondrial membrane composition, improving mitochondrial function. DHA reduced the vulnerability to opening of the mitochondrial permeability transition pore, a pathologic feature of heart failure.67,68
 Clinical Applications
Clinical Applications
Cardiovascular Disease
No other clinical application for fish oil has been as well-studied as cardiovascular disease, including hyperlipidemia, atherosclerosis, stroke, myocardial infarction, and atrial fibrillation. Perhaps because n-3 fatty acids inhibit the development and progression of atherosclerosis and reduce the systemic inflammation that underlies so much of cardiovascular pathology, fish oil has demonstrated benefit for a wide range of applications.69 What has emerged in recent years is the use of the ω-3 index, the sum of EPA and DHA expressed as a percentage of the total fatty acid content of red blood cell membranes, as a potential biomarker for heart disease. It was shown to be inversely associated with the risk for both fatal and nonfatal cardiac events, allowing clinicians to assess individual risk for their patients, and might serve as a tool for monitoring therapeutic efficacy.70,71
For instance, in a study of nearly 800 patients, the ω-3 index was associated with a 69% risk reduction in those with the highest compared with lowest levels for acute coronary syndrome, with an inverse linear relationship between risk and DHA/EPA levels. This amounts to a three-fold greater risk in those with a low ω-3 index than those with a high one. In this study, “low” levels were defined as less than 4%, whereas “high” levels were more than 8%, suggesting a possible therapeutic target.72 In the Heart and Soul Study mentioned previously, those with an ω-3 index above median had a 27% decreased risk of death compared with those with a baseline ω-3 index below median, which was unaffected by adjustment for both traditional cardiovascular risk factors or inflammatory markers.11 This was a prospective cohort study of nearly 1000 patients with stable CHD. Interestingly, in a study published in Journal of the American Medical Association in 2010, a subset of patients recruited from the Heart and Soul Study were found to have an inverse relationship between their ω-3 index and the rate of telomere shortening over a 5-year period.73
Certainly one population that warrants increased intake of DHA and EPA are those with an uncommon (6% of the population) genetic variant that predisposes them to rapid atherosclerotic progression. Individuals with two variants of the ALOX5 promoter have a greater risk for atherosclerosis, marked by an increase in carotid intima–media thickness. Increased consumption of n-6 fatty acids exacerbated this condition, whereas a higher intake of n-3 fatty acids blunted this atherogenic effect.36
Mechanisms of Cardiovascular Benefit
The EPA and DHA in fish oil inhibit the development of atherosclerosis, which can reduce the risk of multiple permutations of cardiovascular disease.56 As discussed in the “Pharmacology” section, fish oil acts through diverse physiologic mechanisms, related to resolution and inhibition of inflammatory pathways, as well as modulation of cellular membrane fluidity. For example, EPA and DHA were shown to increase cell membrane fluidity, affecting intra- and intercellular signaling, and ultimately improving cell function.74,75 EPA and DHA were also shown to stimulate endothelial production of nitric acid, decrease production of inflammatory cytokines and IL-1, and inhibit monocyte migration into atherosclerotic plaques. EPA and DHA also affect gene expression, at least partly via activation of PPARs. For example, in a randomized and double-blinded trial that enrolled over 100 participants, supplementation with 1800 mg/day of EPA/DHA was shown to change the expression of over 1040 genes in peripheral blood mononuclear cells, including genes involved in atherogenesis and inflammation, such as nuclear factor-κβ target genes, proinflammatory cytokines, nitric oxide synthase 3, and genes involved in eicosanoid synthesis.76
In recent years, a number of biologically active compounds derived from EPA/DHA have been elucidated through newly discovered pathways. These are potent mediators of cellular function, with antiinflammatory actions and actions that promote the resolution of inflammation, and are thus known as “resolution-phase interaction products” or resolvins, as well as maresins.77,78 The antiinflammatory component of fish oil may at least partly explain its ability to slow the progression of atherosclerosis, as well as to stabilize atherosclerotic plaques against rupture.79,80 For example, atherosclerotic plaques were shown to incorporate EPA, and a higher EPA content was associated with greater plaque stability, as well as a reduced number of foam and T cells.81
Fish oil also demonstrated lipid-lowering effects, likely mediated partly through the inhibition of VLDL and triglyceride synthesis without reducing production of high-density lipoprotein (HDL) cholesterol.82,83 The triglyceride-lowering effect appears to be dose-dependent, with a greater effect on those with highly elevated triglycerides. This benefit is not seen with plant source oils containing land-based α-linolenic fatty acid–rich polyunsaturated fat sources (e.g., flaxseed oil) or via reduction of saturated animal fatty acids intake.64 In a review of over 40 studies that examined the use of fish oils for either primary or secondary prevention, fish oils were found to reduce the rates of all-cause mortality, cardiac and sudden death, and perhaps stroke.84
As mentioned previously, the AHA for the first time endorsed a nutritional supplement for cardiovascular disease prevention in 2002, advocating consumption of oily fish twice per week among those without heart disease, and at least 1 g/day of EPA/DHA among those with established CAD, and 2 to 4 g EPA/DHA for individuals with hypertriglyceridemia.18 However, there appears to be substantial variability between dietary intake and the ω-3 index (ω-3 blood levels), the latter of which was inversely associated with total mortality among individuals with CAD.11 Optimal intake might be more appropriately determined using the ω-3 index as a benchmark, with current evidence suggesting a target of 8% or higher.85
Elevated Serum Lipids
The most consistent effect of fish oils on serum lipids is a reduction in total triglycerides and VLDL cholesterol, especially in patients with severe hypertriglyceridemia. In general, dietary fish oils appear to both reduce undesirable serum-circulating fats and decrease production of the prothrombotic thromboxanes. In most studies, fish oil supplementation causes a significant reduction of VLDL cholesterol, plasma triglycerides, plasma cholesterol, and LDL cholesterol. In hypertriglyceridemic patients, fish oil supplementation typically results in a significant decline in VLDL cholesterol levels, because large decreases in triglyceride levels lead to a decrease in VLDLs.63,86–89
Recent studies also showed fish oil induced favorable changes in LDL size. It is now well-established that plasma levels of small, dense LDL cholesterol are associated with plasma triglyceride concentrations, are more atherogenic, and are markers for cardiovascular disease risk.90,91 Data from the Quantification of the Optimal n–6/n–3 ratio in the UK Diet (OPTILIP) study indicated that DHA and EPA reduced the proportion of the small, dense LDL cholesterol, even if no changes were seen in total LDL cholesterol levels.92 Data from JELIS, a large-scale intervention trial, found that EPA (1800 mg/day) was particularly effective in patients with the potent risk factor combination of low HDL cholesterol and elevated triglycerides, reducing the risk of CAD in this group by 53%.93
Some studies did not find decreases of total cholesterol or LDL cholesterol after fish oil supplementation.94–97 However, these equivocal studies used olive oil as a placebo, which, as discussed earlier, is a confounder. Additionally, beneficial changes to subfractions and particle size with DHA supplementation (3 g/day) were cited in one randomized trial, despite no significant effect on total LDL cholesterol levels.98 Furthermore, an inverse relationship was found between serum EPA levels and oxidized LDL. Oxidized LDL is emerging as an important biomarker of cardiovascular risk, and is a very early and critical step in the development of atherosclerosis.99,100
For patients with elevated serum cholesterol (>7.75 mmol/L=300mg/dL) or triglycerides (>5.64 mmol/L=500mg/dL), there is sufficient evidence to consider fish oil supplementation of 5 to 10 g/day if lower dosages (2 to 4 g/day) are not effective.101 In one study of 365 patients, supplementation with 10 mL/day (9.2 g) of MaxEPA resulted in significant reductions in triglyceride levels, which were maintained over 4 years.102 Continuing reductions were observed in persons remaining in the study more than 4 years. The authors concluded: “For triglycerides to remain depressed it seemed necessary to maintain a daily intake of 9.2 g MaxEPA (1 g MaxEPA contains 180 mg EPA + 180 mg DHA).”
Fish oil (4 g/day) was also shown to improve the non-HDL cholesterol lowering effect when combined with statin medications. Increases in HDL cholesterol as well as decreases in total cholesterol and triglyceride levels were seen when combined with atorvastatin versus atorvastatin alone.103 Similar results were also documented when used with simvastatin.104
Hypertension
A meta-analysis of over 30 trials (22 double-blinded) investigating the effect of fish oils in reducing blood pressure found that higher doses (median dose 3.7 g/day) were associated with a reduction in systolic blood pressure of 2.1 mm Hg and diastolic blood pressure of 1.6 mm Hg, although lower doses (<0.5 g/day) might not have an effect. The blood pressure lowering effect of fish oil was found to be greater among those aged 45+, as well as among hypertensives versus normotensives.105 A recent review cited a number of mechanisms for a hypotensive effect of fish oil, including changes in eicosanoids, in blood viscosity, in hormonal cellular response, in renin secretion, and decreased response to vasopressors,106 although they also concluded the effect was likely to be small at a dose of 2 to 4 g fish oil per day.
Epidemiologic evidence indicates a hypotensive effect of dietary fish consumption. The largest study is probably the International Study of Macro- and Micro-nutrients and Blood Pressure (INTERMAP) trial, an international cross-sectional epidemiologic study of nearly 5000 men and women living in China, Japan, United Kingdom, and the United States. Researchers found an inverse relationship of total dietary n-3 fatty acids to systolic and diastolic blood pressure, in both hypertensives and normotensives, although the effect was considered small.107
Fish oil seems to have hypotensive effects, ranging from limited (dosages of 5 g/day or less) to substantially larger dosages,51,64,108–115 although there is some conflicting evidence.101 It has been proposed that fish oil depresses vascular response to the hormones involved in hypertension.101 Another suggestion is that fish oil acts by increasing vasodilatory prostaglandins PGI1 and PGI3, and that this increase accounts for observed reductions in blood pressure.101
To test this hypothesis, one study examined the effect of fish oil on blood pressure in men with mild essential hypertension.116 One group received 10 mL of fish oil (3 g of ω-3 fatty acids), a second group received 50 mL (9 g of EPA and 6 g of DHA), a third group received 50 mL of safflower oil (39 g of ω-6 fatty acids), and a fourth group received 50 mL of a mixture of coconut, olive, and safflower oils. The latter group represented the approximate amount and ratio of fatty acids consumed in the average American diet (39% saturated fat, 46% monounsaturated fat, and 15% polyunsaturated fat). The group receiving the highest dose of fish oil (50 mL) had an average reduction of 6.5 mm Hg in systolic pressure and 4.4 mm Hg in diastolic pressure. None of the other groups, including the low fish oil-supplemented group, demonstrated blood pressure reductions in the aggregate. The study did not find the expected association between increased production of PGI1 and PGI3 and sustained reduction in blood pressure. This suggests that vasodilatory prostaglandins are probably not the primary mediators of blood pressure reduction by fish oil consumption, although they may play a role.
Another proposed mechanism is that fish oils may facilitate excretion of sodium and fluid by the kidneys. This was supported by a reported but unpublished double-blind, crossover study in which researchers placed healthy, nonhypertensive men and women on a diet supplemented with approximately 1 g/day of PUFAs for 28 days.117 Each participant received capsules with either fish oil or safflower oil. The fish oil dose was similar to the amount consumed in a single daily serving of tuna, lake trout, or salmon. After 2 weeks, the subjects crossed over to the other supplement. The study found an average 2 to 3 mm Hg drop in both diastolic and systolic blood pressure in those who received fish oil supplements. The researchers also found that fish oil increased urine output by approximately 10%, performing much like a low-sodium diet. This resulted in a reduction in fluid volume. Unlike diuretics, the fish oil supplementation did not increase potassium excretion.
Stroke
In the Health Professional Follow-up Study, a prospective cohort with over 12 years of follow-up, a significantly lower multivariate relative risk (RR=0.57) of ischemic stroke was documented for men who ate fish one to three times a month compared with men who consumed fish less than once a month. Interestingly, higher levels of consumption did not further reduce the risk, and no effect was seen on hemorrhagic stroke.118 A reduction in the risk of ischemic stroke by 30% was also shown in the Cardiovascular Health Study. This trial had nearly 5000 adults aged 65+ years and found a 30% lower risk of ischemic stroke with a fish intake of five or more times a week, and 27% lower risk with a fish intake of one to four times a week, both compared with an intake of less than once a month. In this study, the benefit was limited to tuna or other broiled/baked fish. Consumption of fried fish or fish sandwiches was associated with an increased risk for total and ischemic stroke.119
A very recent study with over 30,000 participants in the United States examined the risk of stroke as it relates to fish consumption. This study examined possible explanations for the increased stroke risk in the “Stroke Belt and Buckle” in the southeastern United States, a region known for having at least a 50% higher incidence of stroke. The authors of this study concluded that regional and racial differences in fish consumption might help to explain this anomaly, i.e., those living in the Stroke Belt are less likely to have two or more servings of fish per week.120
An earlier published in 1995 found that men who ate fish five or more times a week had a 40% lower risk of experiencing a stroke than men who ate fish less than once a week. A similar study in women by researchers at Harvard Medical School found even more impressive results for women.121 In this study, 79,839 female nurses between the ages of 34 and 59 years were followed for 14 years, by which time 574 had experienced a stroke; 303 of the strokes were caused by blood clots, whereas another 181 were caused by a ruptured artery, and the remaining 90 were of undetermined origin. It was determined that women who ate fish once a week lowered their risk of a stroke of any kind by 22%. However, women who consumed fish five or more times per week reduced their risk by 52%. The investigators concluded that women whose intake of fish oils was 0.5 g/day or more had a 30% lower risk of a stroke than women whose intake was less than 0.1 g/day. Of particular interest was the finding that in those women who had a high fish or fish oil consumption, there was no evidence of an increased risk of hemorrhagic stroke. The researchers concluded that the protective effect of fish oils was due to their ability to inhibit platelet aggregation, lower blood viscosity, suppress formation of leukotrienes, reduce fibrinogen levels, reduce blood pressure, and reduce insulin resistance. Curiously, the beneficial effects were substantially more likely in women who did not take aspirin on a regular basis, although subsequent studies have not shown a relationship with aspirin intake.
In a subanalysis of the JELIS trial, a 20% reduction in stroke risk was shown for individuals taking EPA and a statin compared with those only taking a statin, although these results were limited to participants who had already experienced a stroke (i.e., secondary vs primary prevention).10 Currently, more direct intervention trials using fish oil for stroke reduction as the primary outcome are lacking.
Bypass Patients
Some studies demonstrated that fish oil supplementation might help prevent reclosing (restenosis) of the arteries after angioplasty. In one such study, bypass patients were supplemented with 4 g/day of fish oil.122 One year later, those patients who consumed the fish oil supplements had an occlusion rate of 27%, whereas control patients had a 33% rate of occlusion, or a 23% relative improvement. On the basis of a postoperative evaluation 3 weeks after patients had cardiac transplantation, it was found that patients who consumed fish oil supplements had normal endothelium-dependent coronary vasodilation, which remained abnormal in patients who did not consume fish oil.
A much larger and well-conducted recent study published in the Annals of Thoracic Surgery documented a robust protective effect of fish oil after coronary artery bypass grafting (CABG). Of 2100 participants, 44% were given 850 to 882 mg of EPA and DHA as ethyl esters, in the average ratio of EPA/DHA of 1:2. In addition to a lower risk for late mortality (hazard ratio = 0.51 to 0.55), those consuming fish oil had roughly one-half the need for repeat revascularization. Furthermore, they had lower adjusted risk for the composite of death, Q-wave myocardial infarction, or cerebrovascular events. Those with poor left ventricular function had a quite large 64% reduction in mortality when taking fish oil.123 The authors of this study supported the use of fish oil supplementation for all patients as part of standard medical therapy after CABG.
Atrial Fibrillation
A link has long been suspected between fish oil consumption and atrial fibrillation based upon epidemiologic and experimental evidence, although the benefit is not clearly established. In the prospective cohort study (described previously in the “Stroke” section) that found a reduction in the risk of stroke with broiled or baked fish, a roughly 30% lower risk of atrial fibrillation was also found for those consuming fish regularly, although not fried fish.124 The association between fish oil intake and reduced fatal CHD and sudden cardiac death also lent strong support for its use in preventing ventricular arrhythmias.125
(1) modulation of electrophysiologic and metabolic heterogeneities secondary to atherosclerotic disease,
(2) modulation of cardiac myocyte metabolic activity and cardiovascular oxidant stress,
(3) direct modulation of ion channel and transporter activity,
(4) indirect modulation of ion channel and transporter activity, via modulation of autonomic nervous system activity, and
(5) modulation of inflammatory pathways that promote ectopic electric activity and abnormal conduction.126
Also, atrial fibrillation is known to occur more frequently in damaged hearts, which may contribute to mitochondrial pathology and insufficient adenosine triphosphate production, an effect possibly modulated by fish oil.127
However, not all epidemiologic studies found a protective effect, including the Rotterdam study, and the Danish Diet, Cancer, and Health Study, two very large prospective cohort studies.128,129 Most recently, the Women’s Health Initiative, a large cohort of healthy women, also reported no apparent benefit of increased fish consumption.130
A recent meta-analysis of randomized clinical trials published in the journal Heart also did not find benefit for the prevention of atrial fibrillation. However, this study had important limitations, including significant heterogeneity and methodologic flaws in the included trials, and fairly limited statistical power to detect a benefit. Its authors also acknowledged that most of the studies used a dose that might have been too low (3 to 4 g/day fish oil) to have a clinical benefit.131 A randomized and placebo-controlled study published recently in Journal of the American Medical Association also found no benefit when giving 8 g of prescription ω-3 fatty acids (1 g prescription ω-3 fatty acids contains 375 mg of DHA and 465 mg of EPA), during the first 7 days and 4 g/day for 24 weeks.132
It is thus difficult to determine the role fish oil has on incidence as well as the recurrence of atrial fibrillation, given the conflicting evidence published so far. It may be that optimal administration, dosage, etc., play a role, but given the complexity underlying atrial fibrillation, it may also depend on the specific etiology and the timing. For example, some data suggested that fish oil was more likely to be of benefit if CAD was also present for ventricular arrhythmias, whereas those without heart disease were unlikely to benefit.133
Not all atrial fibrillations have the same pathology, with different pathophysiologic mechanisms characterizing distinct clinical presentations. Some authors suggested that n-3 fatty acids might be more likely to be of benefit for preventing the structural remodeling that leads to resistant and/or permanent atrial fibrillation, which is more likely with underlying heart conditions or after myocardial infarction.134
In summary, doses used clinically so far may not be sufficient for benefit, and not all presentations of atrial fibrillation may respond to treatment. Earlier treatment with fish oil is more likely to have an effect, before significant structural remodeling has taken place. Furthermore, given the mechanisms involved with fish oil, a longer time until benefit is seen might be expected, an effect not considered in many trials. For example, in the randomized trial cited previously by Kowey et al132 almost half of recurrences occurred within the first 2 weeks of follow-up (of 24 total), suggesting insufficient time for the build up of tissue levels.
Myocardial Infarction and Acute Coronary Syndrome
One of the largest trials to document a protective benefit for individuals following a myocardial infarction was the GISSI study.135 In this large secondary prevention trial, 1 g of n-3 fatty acids (containing 850 to 882 mg EPA/DHA ethyl esters) greatly reduced sudden cardiac death within 4 months of starting therapy, as well as an all-cause and cardiovascular reduction in mortality. The reduction in the incidence of sudden cardiac death accounted for about 57% of the total improvement in mortality rates. At the end of the study, 2.7% of the placebo group participants died from sudden cardiac death compared with 2% in the fish oil group. Overall, cardiovascular death (including stroke) at the end of the study was 6.5% in the placebo group versus 5.5% in the fish oil group. There was no statistically significant difference in the incidence of nonfatal heart attacks between the fish oil and placebo groups. The researchers concluded that fish oils exerted their protective effect by preventing fatal ventricular arrhythmias rather than through an improvement in cholesterol profile. They did note a small drop in triglyceride levels of 4.6% in the fish oil group but found no significant differences in LDL and HDL cholesterol between the two groups. They also pointed out that the number of lives per 1000 patients that could be saved every year by giving heart attack survivors fish oil exceeded the number of lives per 1000 patients estimated to be saved by treating heart disease patients with high cholesterol levels with pravastatin. This puts fish oil supplements squarely in the category of a highly effective unpatentable heart drug, although now, Lovaza, a prescription-only form of fish oil is available.
Furthermore, a second trial with over 9000 participants from the GISSI study found that treatment significantly reduced sudden death, with greater benefit in those with left ventricular systolic dysfunction (four-fold greater risk reduction), which increases the risk for mortality.136
The JELIS trial, a prospective trial that combined 1800 mg EPA with a statin for hypercholesterolemic patients, found a 51% reduction in risk for myocardial infarction or cardiac death over 5 years. A reduced risk for myocardial infarction was found for those with and without a previous myocardial infarction, although the effect was greater in those with the former. This study was particularly important because they were able to examine adherence to therapy, and found a greater effect in those with complete adherence.137,138
However, more recent studies did not find benefit for survivors of myocardial infarction in terms of reducing the rate of major cardiovascular effects. For example, a double-blinded, randomized trial with nearly 5000 participants reported no benefit with 400 mg/day of EPA+DHA. Participants in this study were also treated aggressively with other antihypertensive, antithrombotic, and lipid-modifying therapies, which greatly reduced their risk for subsequent cardiovascular events, which might have reduced any potential benefit of fish oil therapy. Additionally, this study was criticized for using too low of a dose of fish oil for a clinically meaningful effect.40 Similarly, the German OMEGA trial (a randomized, placebo-controlled trial to test the effect of highly purified ω-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction), which also used aggressive therapies and a fairly low dose of fish oil (460 mg EPA, 380 mg DHA) did not report a therapeutic effect.139 The authors of this study reported limitations, including its low power to detect benefit, given the risk reduction associated with other therapies. Also, they reported that both groups (those receiving fish oil and those not) significantly increased their dietary fish consumption during the study period. Given the low dose used, it was not clear that those receiving supplements had a significantly higher intake of EPA/DHA than those who did not.
Considerable animal based and experimental evidence showed that dietary fats modulate the electrical stability of the myocardium, and that the appropriate fatty acids can reduce the vulnerability to arrhythmia during myocardial ischemia.140–142 Raising the ω-3 fatty acids to higher levels in the myocardium may also prevent postinfarction ventricular fibrillation.143–145 Recent data in humans with acute myocardial infarction found that 465 mg/day EPA and 375 mg/day DHA improved ultrasound indexes of endothelial function without affecting serum asymmetric dimethylarginine levels, suggesting preclinical benefit.146
One early study (DART) evaluated the effect of dietary intervention in 2033 men recovering from myocardial infarction.4 Patients were randomly allocated to receive one of four types of dietary advice:
1. Lowered intake of dietary fat
2. Consumption of at least two portions of fatty fish (200 to 400 g) a day
3. Supplementation with three capsules (1.5 g) of MaxEPA (1 g Max EPA contains 180 mg EPA + 180 mg DHA) a day
At the end of 2 years, those in the group consuming fish were found to have increased their EPA intake to four times that of other subjects. This group also experienced significantly lower mortality. The fat and fiber advice groups showed no differences in mortality. Although the rate of recurrence of heart attack was similar in all groups, the fish and fish oil group had a 29% reduction in risk of death compared with the other groups. These findings are in direct conflict with an earlier study showing no such benefits.147 However, it may be significant that the earlier study was conducted over a short period of 6 weeks.
Even in patients with no apparent clinical evidence of arterial disease, fish consumption was shown to improve arterial wall function. One study found that in both healthy and non–insulin-dependent diabetes mellitus patients, those who ate fish showed significantly better compliance of their left subclavian artery and femoral arteries.148
Fish and fish oil intake were also associated with the risk for angina and acute coronary syndrome. In one case–control study, individuals with acute coronary syndrome were found to have a 20% lower EPA/DHA content in their red blood cell membranes than controls. Those with the highest levels had a nearly 70% lower risk for acute coronary syndrome than those with the lowest levels.72 Another recent epidemiologic study suggested that the benefit of dietary fish consumption might be limited to fatty fish (i.e., not lean fish), with a more apparent benefit in men.149 Long-term fish consumption might be particularly protective, providing another rationale for fish oil use in those with increased cardiovascular risk.150
Interestingly, one study of men with acute coronary syndrome found that symptoms of depression increased as blood levels of EPA/DHA decreased, supporting a possible link between depression and cardiovascular outcomes.151 Given the underlying pathophysiology, this seems quite plausible from a systems biology perspective.
A meta-analysis published in the Annals of Medicine in 2009 found an overall 29% and 23% reduction in risk for cardiac death and all-cause mortality, respectively, when using fish or fish oil. In patients with a previous myocardial infarction, the reduction in risk was even greater (57%). Surprisingly, they found an increased risk for sudden cardiac death in patients with angina with higher fish/fish oil intake.152 This increased risk for participants with angina was unexpected, because previous studies showed that fish oil supplementation reduced the number of angina attacks and reduced mortality in men recovering from myocardial infarction.4,153 Significant rheologic improvements in patients with stable angina pectoris might occur after daily fish oil supplementation with 2.8 g of EPA and 1.8 g of DHA.154 In a double-blind, placebo-controlled study, fish oil supplementation resulted in increased red blood cell deformability, reduced whole blood viscosity, and prolonged bleeding time compared with olive oil supplementation. The frequency of angina attacks decreased in both groups. However, neither type of oil affected exercise capacity or hemodynamic response to exercise.
Finally, as mentioned previously, a comprehensive review of use of fish oils for either primary or secondary prevention concluded that they reduced the rates of all-cause mortality, cardiac and sudden death, and perhaps stroke.84 Additionally, an analysis of over 15,000 patients from the JELIS trial published in 2011 in the Journal of Atherosclerosis and Thrombosis found a significant reduction in major coronary events, defined as sudden cardiac death, fatal or nonfatal myocardial infarction, unstable angina pectoris, and angioplasty/stenting or coronary artery bypass grafting. Those with higher plasma levels of EPA (participants were supplemented with EPA only, not DHA) had the greatest reduction in risk.155
Immune Function
Several of the mechanisms by which fish oil modulates immune function have now been clarified, although much remains to be determined, including optimal dosing and proportions of DHA/EPA necessary for a clinical effect. Additionally, how in vitro or ex vivo changes translate into clinical significance has not been well established. In healthy subjects, the effect of EPA and DHA on ex vivo lymphocyte proliferation was reported in at least 14 articles, at 27 different dose levels, ranging from 0.2 to 7 g EPA+DHA per day, whereas the influence on cytokine production by monocytes was evaluated in 24 studies in 46 treatment cohorts.156 It was shown that increasing the EPA/DHA content of immune cells affected a number of immune functions, and that DHA and EPA had varying effects. For example, in one double-blinded study of healthy volunteers, a marker of T-lymphocyte activation was inhibited by approximately 5 g/day of DHA, whereas EPA and olive oil had no effect.157 Most studies, however, did not show any effect on lymphocyte activation, although differences were cited between different age groups and genders. Similarly, although some reports suggested an inhibitory effect on natural killer cell function and/or modulation of cytokine production by lymphocytes, there was considerable inconsistency among the studies, and no clear relationship exists.156
Per an excellent review published in 2007, the greatest modulation of immune function by fish oil was mediated via alterations in inflammatory cytokine production by monocytes, including IL-1β, TNF-α, and IL-6.156 This ties in with an increased awareness of the links between inflammatory and immune function. Even here, however, there are inconsistencies. Not all studies documented an inhibitory effect (although none showed an increase in inflammatory cytokine production), and the expected dose-dependent relationship was not always observed. One very plausible hypothesis for these inconsistencies is that highly relevant genetic polymorphisms are often not taken into account, such as those that affect TNF-α production.158
Decreased production of the proinflammatory mediator PGE2, as well as the incorporation of EPA and DHA into human inflammatory cells does appear to follow a dose-dependent relationship, along with an inverse relationship between EPA and TNF-α and IL-1β in most studies. One study suggested that at supplementation levels of 1.65 g EPA+DHA per day, no effect was seen, and a threshold existed between 1.65 and 3.3 g EPA+DHA per day.159 Data also suggested that older individuals, as well as those with specific inflammatory conditions, might experience a greater therapeutic effect of fish oil than young healthy individuals.
Some research also suggested that a higher n-6:n-3 ratio has an inhibitory effect on phagocytic function, suggesting higher intake of n-3 fatty acids might improve this activity.160 This was confirmed in one trial of healthy volunteers given 3 g/day fish oil (26% EPA and 54% DHA), which found an increase in phagocytic activity of 62% and 145% in neutrophils and monocytes, respectively.161 Very recent data described resolvin D1 (derived from DHA) recognition sites on phagocytes (G-protein coupled receptors), which mediate its actions, leading to enhanced phagocyte and clearance functions, as well as resolution of acute inflammation.162 Research published in 2009 in Nature also pointed to critical immune roles for other resolvins related to microbial sepsis and immune vigilance.163
Autoimmune and Inflammatory Diseases
Fish oils may play a role in the treatment of autoimmune disease (e.g., SLE, dermatomyositis, autoimmune nephritis, multiple sclerosis, celiac disease)164–167 and inflammatory disorders (e.g., RA,168 psoriasis,169 atopic dermatitis1). This may be in part due to the chronic inflammation that often plays a role in the development and perpetuation of these conditions. For example, DHA may directly inhibit the release of AA in intestinal epithelial cells when stimulated by gliadin.170 Furthermore, fish oils may help to improve the comorbidities that often accompany autoimmune disease, such as the premature incidence and acceleration of atherosclerosis associated with SLE. Clinical trials in humans demonstrated a benefit not only for disease activity, but also for improving markers of cardiovascular function. In one trial, patients with SLE taking 3 g MaxEPA (1g Max EPA contains 180 mg EPA + 180 mg DHA) had a significant reduction in disease activity (measured by Systemic Lupus Activity Measure [SLAM-R]171). In a second randomized and double-blinded, placebo-controlled trial, participants given Omacor (1.8 g EPA and 1.2 g DHA per day) showed significant improvement in two markers of disease activity (British Isles Lupus Assessment Group and SLAM-R). Additionally, markers of endothelial function and oxidative stress improved after therapy.172 Importantly, this study used olive oil as a placebo, which also showed benefit for several cardiovascular risk factors, yet fish oil provided a statistically significant benefit in comparison.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is in many ways a representative chronic inflammatory autoimmune disorder, and may serve as a model for other conditions. The enzyme COX-2 is overexpressed in the synovium of patients, as are products of the enzyme 5-LOX. Interestingly, a study from Spain found decreased levels of ω-3 fatty acids in the blood and synovial fluids of male and female RA patients compared with healthy controls.173 Epidemiologic evidence suggested that RA might be linked to n-3 fatty acid intake. For example, it is rare among Eskimos, yet by comparison, 2% of the world’s population is affected. Studies of the Japanese population confirmed an inverse relationship between high dietary fish consumption and a low incidence of RA.174 Further support comes from a population-based, case–controlled study in women that found a decreased risk of RA in those who consumed the most fish.168
A number of reviews concluded that fish oil supplementation was associated with a number of benefits; it reduced pain, the number of tender joints, the duration of morning stiffness, as well as the use of non-steroidal antiinflammatory drugs (NSAIDs) in patients with RA, and was associated with improved physical performance.175 A meta-analysis of 17 randomized controlled trials published in 2007 in the journal Pain supported these conclusions, although the authors concluded that it might take several months of therapy for an effect.176 This slow building effect might also explain results of an earlier study that found the anti-inflammatory effect of the fish oils continued for up to 4 weeks after cessation of supplementation.
The reduction in the use of NSAIDs is no small benefit. Growing concern about the adverse effects and safety of long-term NSAID use makes any therapy that reduces their necessity quite attractive. For example, in an extremely large meta-analysis published in 2011, every single NSAID evaluated, including ibuprofen and celecoxib, was associated with an increased risk for cardiovascular death.177 In one small but well-controlled study, cod liver oil was able to reduce NSAID use more than 30% in 39% of patients versus only 10% of controls.178 Fish oil was also shown to enhance the COX inhibiting effect of other medications, specifically of paracetamol, offering additive clinical benefit.179 Similar benefit for reducing disease activity was also cited for indomethacin.180
Interestingly, in a recent review of the efficacy of fish oil for RA, researchers looked at the combination of benefits, including a reduction in symptoms, decreased use of medications, and improvement in comorbidities, and concluded that an argument for the use of fish oil in RA is strong.181 However, they also concluded that:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


