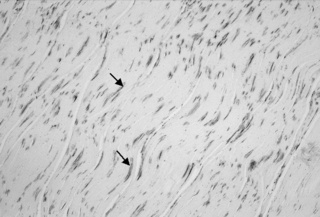
4.2 Fascia is alive In an examination of human lumbar fascia, a group of biomechanical investigators around Yahia et al. (1993) discovered its ability for tissue contraction. Three years later, the German anatomy professor Staubesand, in an examination of the human fascia profunda of the lower leg, documented the presence of smooth muscle-like cells (Staubesand & Li 1996). As he also found a rich presence of sympathetic nerve fibers in their vicinity, he postulated a potential close connection between sympathetic activation and fascial tonus regulation. Indeed, many clinicians report a frequent association between long-term psychological stress and a perceived increase in palpatory myofascial stiffness. Such increase in tissue stiffness seems to be present also at rest, a condition for which most electromyography experts agree that most skeletal muscles are electrically silent (Basmajian & DeLuca 1985). It has therefore been suggested that human resting muscle tone may be significantly influenced by changes in fascial stiffness (Masi & Hannon 2008). Unexpectedly, it was also revealed that the intramuscular perimysium seemed to express a higher density of myofibroblasts than the endomysium, perimysium, or fascia profunda. Interestingly, meat scientists report that tonic muscles tend to contain a thicker perimysium, giving them the appearance of tough meat (in contrast to the tender meat quality of phasic muscles, which have a much thinner perimysium; Borg & Caulfield 1980). It has therefore been suggested that the augmented resting stiffness of some tonic muscles could be related to an enhanced myofibroblast density in their perimysium (Schleip et al. 2006b). In some tissue samples of the lumbar fascia a dramatically increased density of myofibroblasts was found. Density was then comparable to that reported in Dupuytren contracture or frozen lumbars. This could suggest that the lumbar fascia may sometimes express a pathological condition similar to those two common fascial tissue contractures (Fig. 4.2.1). Fig. 4.2.1 • Indications for fascial pathology of “frozen lumbar”? Section of the posterior layer of the lumbar fascia at the level of L2. Note the high density of myofibroblasts, which in this patient is comparable to their reported density in the shoulder capsule during a “frozen shoulder” pathology. This suggests that, at least in this patient, the low back region could be affected by fascial contracture and stiffening similar to that of a “frozen shoulder”. Arrows indicate examples of stress fiber bundles containing α-smooth muscle actin (a differential marker for myofibroblasts), which are stained here in dark gray. Length of image 225 μm. Adapted from Schleip et al., 2006a, with permission. It has been proposed that a tendency for high myofascial stiffness might be a polygenic human trait, being associated with a predisposition for living in colder climates (Masi & Hannon 2008). This is supported by the high prevalence of ankylosing spondylitis and Dupuytren contracture in people with a Northern European ancestry line. On the other hand, the condition of general joint hypermobility is more frequently expressed in people from Africa and Southern Asia. It has therefore been suggested that general joint mobility (and tissue stiffness) could be influenced by myofibroblast density in muscular fasciae (Remvig et al. 2007). This would be congruent with the finding that hypermobile people tend to have a slower wound contracture and reduced scar formation, whereas people with “Vikings’ disease” (i.e., Dupuytren contracture) tend to have faster wound contracture, are more prone to scarring, and are also more often affected by other myofibroblast-driven fascial contractures, such as frozen shoulder or plantar fibromatosis (Hart & Hooper 2005). It is assumed that most myofibroblasts develop out of regular fibroblasts. This transition is stimulated by an increase in mechanical strain, as well as by specific cytokines Figure 4.2.2. Myofibroblasts play an important role during wound healing, and are also involved in many pathological fascial contractures (such as Peyronie’s disease, hypertrophic scar, plantar fibromatosis, Dupuytren contracture, or frozen shoulder). Due to their possession of dense ASMA stress fiber bundles, their contractile capacity is four times stronger than regular fibroblasts. Fig. 4.2.2 • Two states of myofibroblast differentiation
How cells modulate the tonicity and architecture of fascial tissues
Fascial tonicity
From myofibroblast contraction to tissue contractures
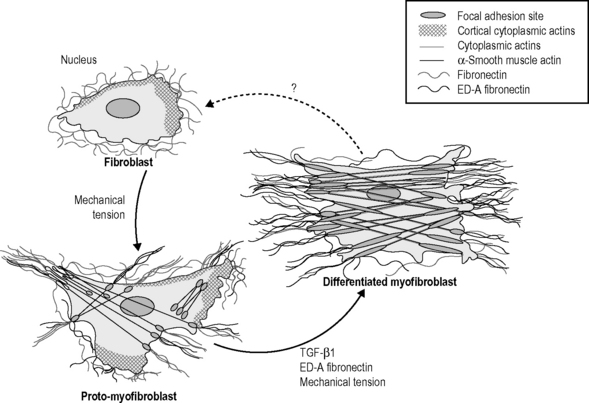
In vivo, fibroblasts might contain actin in their cortex but they neither show stress fibers nor do they form adhesion complexes with the extracellular matrix. Under mechanical stress, fibroblasts will differentiate into protomyofibroblasts, which form cytoplasmic actin-containing stress fibers that terminate in fibronexus adhesion complexes. Protomyofibroblasts also express and organize cellular fibronectin ? including the ED-A splice variant ? at the cell surface. Functionally, these cells can generate contractile force. TGF-β1 increases the expression of ED-A fibronectin. Both factors, in the presence of mechanical stress, promote the modulation of proto-myofibroblasts into differentiated myofibroblasts that are characterized by the de novo expression of α-smooth muscle actin in more extensively developed stress fibers and by large fibronexus adhesion complexes (in vivo) or supermature focal adhesions (in vitro). Functionally, differentiated myofibroblasts generate greater contractile force than protomyofibroblasts, which is reflected by a higher organization of extracellular fibronectin into fibrils. From Tomasek et al., 2002, with permission.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine






