Slightly greater incidence in males than females.
Age
The first two decades of life account for 80 % of patients (age at diagnosis ranges from 13 to 19 years), being more uncommon in adults and elderly patients and more frequent in white (Caucasian) people.
Sites of Involvement
Most frequently osseous in origin (Figs. 25.1, 25.2, and 25.3), but around 10 % of cases arise in extraskeletal soft tissues.
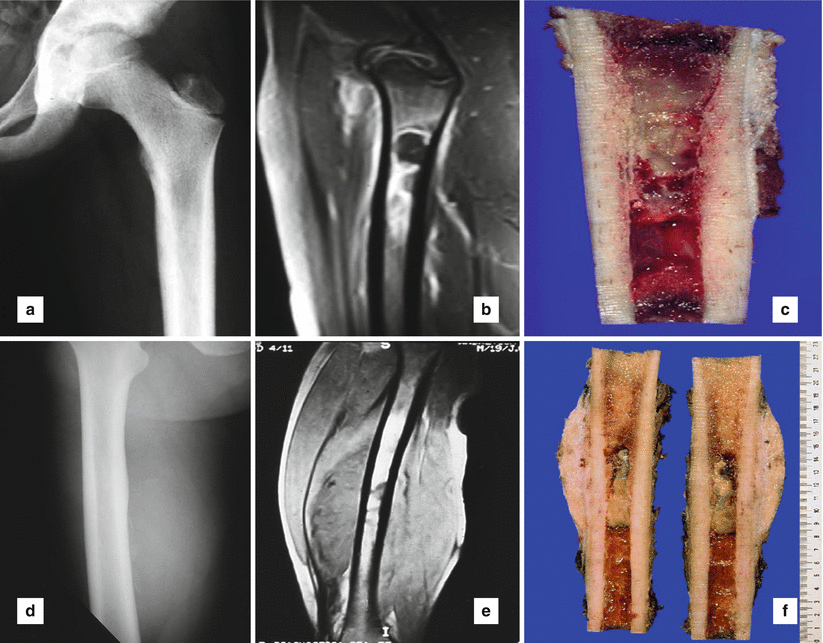
Fig. 25.1
Ewing’s sarcoma with osteolytic lesion involving a long tubular bone, upper metaphysis of a femur, without extension into soft tissue: (a) conventional radiography, (b) magnetic resonance imaging (MRI), (c) grossly intramedullary tumor location. Ewing’s sarcoma with osteolytic lesion in upper femoral shaft, and extension into soft tissue: (d) conventional radiology, (e) MRI, (f) macroscopically, intramedullary location with cortical, periosteal, and soft tissue extension
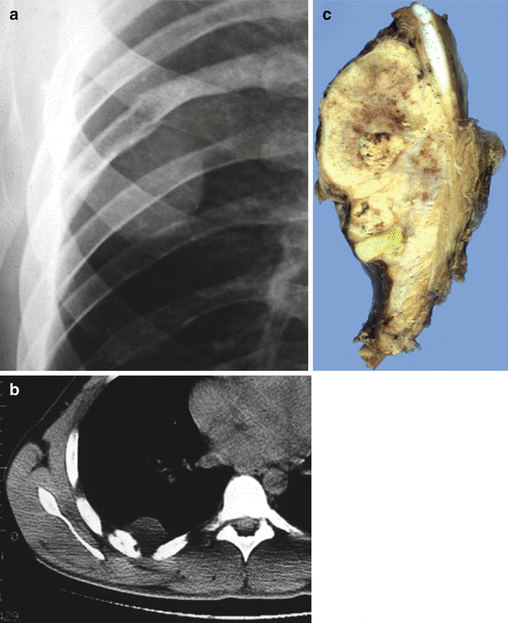
Fig. 25.2
Costal Ewing’s sarcoma with osteolytic lesion and extension into soft tissue (Askin tumor). (a) Conventional radiography, (b) computed tomography (CT scan), and (c) grossly large mass with bone and extensive soft tissue involvement
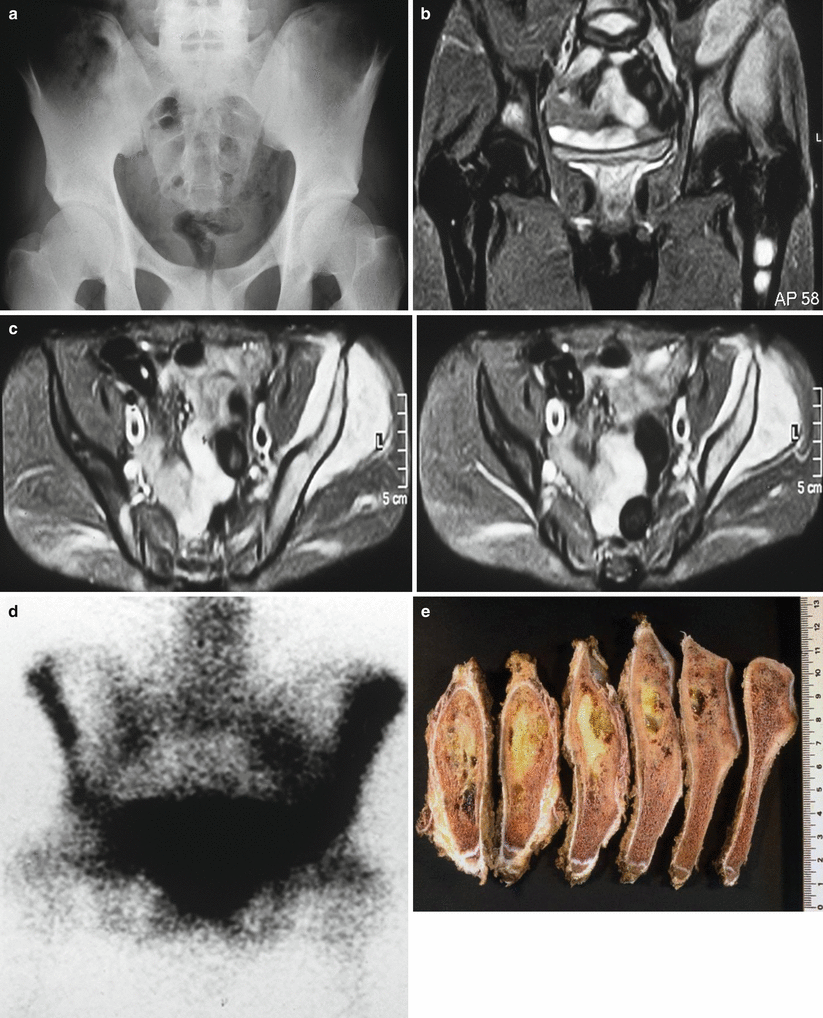
Fig. 25.3
Pelvis Ewing’s sarcoma with flat bone involvement and extension into soft tissue area: (a) conventional radiology, (b and c) MRI, (d) bone scan, (e) macroscopic iliac tumor infiltration
Extraskeletal location can be present as an extension (Fig. 25.1f) from a primary bone tumor (parosteal or periosteal location).
Predilection for the diaphysis or metadiaphysis of long tubular bones (Fig. 25.1a–c) and flat bones mainly of the pelvis (Fig. 25.3) and ribs.
Can affect appendicular or axial bones.
Occasional superficial location.
Clinical Symptoms and Signs
Pain and enlarging mass associated with swollen soft tissue.
Systemic symptoms.
Pathologic fracture is infrequent.
There is a need to improve diagnostic tests to identify Ewing’s sarcoma.
Image Diagnosis
Radiographic Features
No features specific for ESFT.
Indefinite osteolytic lesion mainly involving the diaphysis of a long tubular bone with or without extension into adjacent soft tissue areas (Figs. 25.1a, 25.2a, and 36.3a).
Appearance is variable, fluctuating from osteolytic (Fig. 25.1a) to sclerotic, although lytic bone lesions predominate with moth-eaten permeative features.
Laminated or multilayered periosteal reaction (onion skin-like) pattern (Fig. 25.10). Less frequent perpendicular (sunburst type) reactive bone.
Saucerization of bone causes a concave expansion of the long tubular bones in periosteal tumors.
Tumors reduce in size in some cases after therapy/chemotherapy (Fig. 25.9).
CT Features
Destructive intraosseous tumor with soft tissue extension (Fig. 25.2b).
Soft tissue calcification is uncommon.
MRI Features
Image Differential Diagnosis
Osteosarcoma
Medullary and cortical bone destruction, aggressive periosteal reaction (Codman triangle); tumor matrix ossification is variable depending on the amount of bone tumor formation, calcified matrix, and osteoid production.
Osteomyelitis
The earliest changes are seen in adjacent soft tissues with swelling and loss of normal fat planes. An effusion may be seen in an adjacent joint. Periosteal reaction and periosteal thickening are variable. CT is better for identifying a sequestrum.
Metastasis
Metastatic lesions can have almost any appearance. They can mimic a benign lesion or an aggressive primary bone tumor. The appearance on CT will depend on the degree of mineralization of the metastasis. It can be a lytic or sclerotic lesion.
Hemolymphoid Tumors (Lymphoma, Leukemia, Langerhans Cell Histiocytosis)
Solitary or multiple punched-out lytic lesions with or without sclerotic rim
Pathology
Gross Features
Intramedullary location is frequent with a tan-white or tan-gray hemorrhagic mass with cortical, periosteal, and soft tissue extension (Fig. 25.1c).
Irregular and destructive bone cortex with subperiosteal nests and periosteal elevation.
Soft tissue extension can be present extending bone margins and exceeding intraosseous component (Figs. 25.2d and 25.3d). Soft tissue tumor mass larger than 10 cm in size favors a poor prognosis.
Post chemo- or radiotherapy, the neoplasm may reabsorb.
Histological Features
Diffuse proliferation of small round cells fluctuating between undifferentiated patterns (conventional Ewing’s sarcoma) (Fig. 25.4a) and histology deserving neuroectodermal differentiation with Homer-Wright pseudorosettes (so-called PNET) (Fig. 25.4b). Presence of atypical variants mainly based upon cell size.
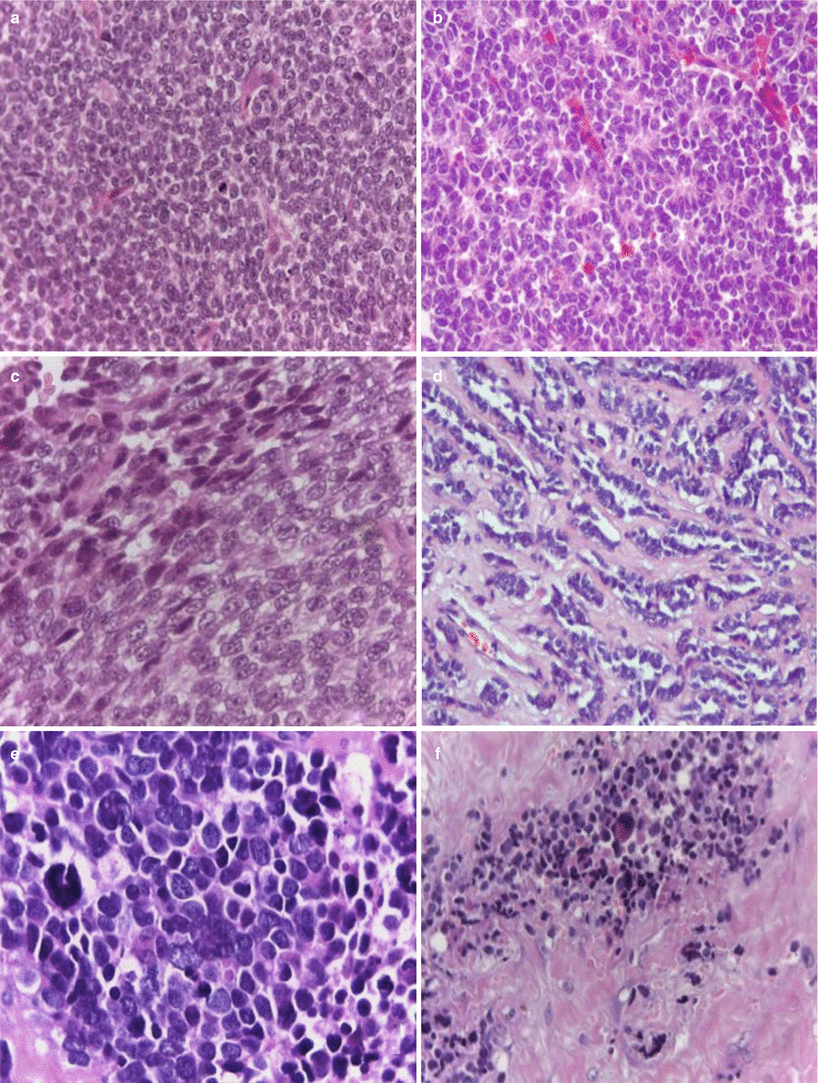
Fig. 25.4
(a) Conventional Ewing’s sarcoma, hematoxylin/eosin (H&E) 20×, (b) PNET with Homer-Wright pseudorosettes formation, H&E 20×; (c) atypical Ewing’s sarcoma with spindle cell pattern, H&E 40×; (d) atypical Ewing’s sarcoma (adamantinoma-like) variant H&E 20×; (e) atypical Ewing’s sarcoma (large-cell variant) H&E 40×; and (f) sclerosing Ewing’s sarcoma, H&E 20×
Small round cells with round to oval nuclei, inconspicuous nucleoli, and scant, eosinophilic, or clear cytoplasm. Also denominated: “small round blue cell tumor” because of the intense H/E dark nuclear staining.
Stroma can be scant or fibrotic or with sclerosis and lace-like appearance. Rich neoangiogenesis and hemorrhagic lacunae (vascular Es/PNET).
Geographic necrosis is frequent, and apoptosis/mitosis varies from case to case.
Mainly the tumor cells display abundant PAS-positive glycogen, but occasional cases are negative (10 %).
Three larger categories appear according to their predominant morphological criteria: conventional/classical/typical ES, PNET with neuroectodermal features, and atypical ES comprising subtypes distinct from the other two types.
Pathologic Differential Diagnosis
Small-Cell Osteosarcoma
Major cell heterogeneity and osteoid matrix (Fig. 25.7a) production with focal lace-like osteoid.
GAL1 and SATB2 immunoexpression. Osteonectin positive (quite unspecific).
Possibility of EWSR1 rearrangement has been communicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








