Abstract
Objective
The aim of this study was to evaluate the effectiveness of injecting botulinum toxin A into the lower limbs of children with cerebral palsy, according to age, dose, dilution, injection site and needle placement technique (manual or ultrasound guidance).
Materials and methods
Any child with cerebral palsy examined between May 2005 and May 2006 who needed botulinum toxin A injections in the adductor, hamstring, gastrocnemius and/or soleus muscles could be included. Fifty-four (54) children participated in the study, 30 of whom were injected under ultrasound guidance. The pre- and post- toxin evaluations were done through analytical clinical examination and the Gross Motor Function Measure (GMFM-88).
Results
We found an overall clinical effectiveness for 51% of the children. This effectiveness was significantly higher for children under 6 years old or over 12, especially when the doses were greater than 0.8 UI/kg per muscle of Botox ® , when the injected muscles were hamstrings or gastrocnemius, and when the injections were guided by ultrasound. Dilution had no effect on clinical effectiveness. Function after one month was better for 24% of the children. This functional improvement was significantly better for children under 6 years old with the injections under ultrasound control.
Conclusions
This study confirms that the effectiveness of botulinum toxin injections is higher in younger children, with injected doses higher than 0.8 UI/kg per muscle of Botox ® and injections guided by ultrasound.
Résumé
Objectif
Évaluer l’efficacité des injections de toxine botulinique A en fonction de l’âge des enfants infirmes moteurs cérébraux, de la dose injectée, de la dilution, des sites injectés et de la méthode de repérage (clinique ou sous échographie).
Matériel et méthode
Tout enfant suivi entre mai 2005 et mai 2006 présentant une infirmité motrice cérébrale nécessitant l’injection de toxine botulinique au niveau des adducteurs, ischio-jambiers, gastrocnémiens et/ou soléaires pouvait être inclus. L’évaluation pré- et post-toxine se faisait sur l’examen clinique analytique et par l’Évaluation motrice fonctionnelle globale 88 (EMFG-88).
Résultats
Cinquante-quatre enfants inclus. Trente enfants ont été injectés sous échographie. L’efficacité clinique globale est de 51 %. Elle est significativement meilleure chez les enfants de moins de six ans et plus de 12 ans, lorsque la dose est supérieure à 0,8 UI/kg par muscle de Botox ® , lorsque les muscles injectés sont les ischio-jambiers ou les gastrocnémiens et lorsque le repérage est réalisé sous échographie. La dilution du produit n’a pas d’effet sur l’efficacité clinique. La fonction est améliorée à un mois chez 24 % des enfants. L’amélioration fonctionnelle est significativement meilleure chez les moins de six ans, et lorsque le repérage s’est fait sous échographie.
Conclusion
Cette étude confirme la meilleure efficacité de l’injection de toxine botulinique chez les enfants les plus jeunes, injectés à des doses supérieures à 0,8 UI/kg par muscle de Botox ® et sous repérage échographique.
1
English version
1.1
Introduction
Since the first publications about using botulinum toxin in 1993 , it has become increasingly common to use the botulinum toxin A to treat lower limb spasticity in children suffering from cerebral palsy. Even though the French Drug Marketing Authorization (AMM) currently only authorizes the injection of the muscles responsible for the dynamic deformation of club foot, most teams now typically inject other muscle groups than the triceps surae, frequently with multi-site injections . A regular evaluation of this practice is necessary.
Intramuscular injections of botulinum toxin are generally guided clinically . However, this manual procedure can be insufficient to insure correct needle placement for muscle injections, even in superficial muscles. Chin et al. found poor needle placement in 22% of the gastrocnemius injections in children . Clinical guidance of injections is even more difficult with young children, whose muscles are sometimes quite thin. Electromyographical guidance or electrical stimulation, improves the precision of the injection in adults, but its use with children is limited due to the pain it engenders and/or to the sometimes tricky cooperation of the patients . When dealing with multi-site injections, some teams perform the injections under general anesthesia to limit these problems . However, this tactic cannot be used regularly without risk to the children, who often need numerous injections sessions during the growth period.
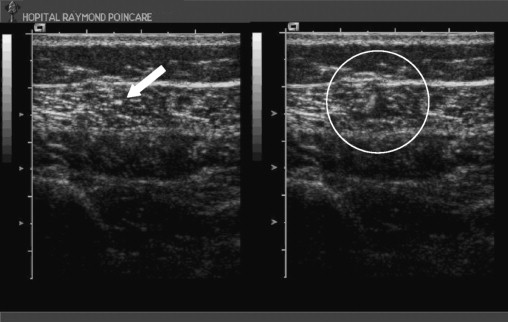
Ultrasound guidance seems to be a good alternative for use with children who have cerebral palsy: it allows the identification of the muscle to be injected and the verification of the position of the needle tip in the designated muscle mass ( Fig. 1 ). To our knowledge, none of the studies thus far published have investigated the advantages of this technique for injecting botulinum toxin into the lower limbs of cerebral palsy patients, if not for deep muscle injections (e.g., the ilio-psoas muscle ).
In this article, we report the results of a prospective study whose objective was to evaluate the clinical and functional effectiveness of botulinum toxin injections for the treatment of spasticity in the lower limbs of children with cerebral palsy, in terms of the use or non-use of ultrasound guidance.
1.2
Materials and methods
1.2.1
Study population
During the period from May 2005 to May 2006, all the ambulatory children examined in outpatient consultations in the spastic diplegia service, consequential to periventricular leukomalacia lesions for which the examining doctor recommended an injection of botulinum toxin in the lower limbs, were seen by the senior doctor in the service who, after a clinical examination, confirmed or rejected the examining doctor’s recommendation and suggested injection sites. The injection site could be a single or multiple. The exclusion criteria were: children who had surgery less than three months previous, and children who were not re-evaluated one month after their surgery due to distance concerns.
1.2.2
Evaluation methods
In order to determine the injection sites, every child had a visual gait examination and an analytical clinical examination to evaluate the muscular force, the spasticity on the Tardieu scale , and the articular amplitudes according to the Varax group .
A functional evaluation was done using the Gross Motor Function Measure (GMFM-88), which includes a scale of 88 items, divided into five categories:
- •
A: lying and rolling;
- •
B: sitting:
- •
C: crawling and kneeling;
- •
D: standing;
- •
E: walking, running, and jumping.
The children were re-examined clinically and functionally 3 to 4 weeks after the injections. During this period, the re-education process continued at the same rhythm as before the injection(s). Others continued to be worn, day and/or night, also at the same rhythm as before the injection(s). During the examination, the parents were asked if their child had had pain at the injection sites or if their child had experienced reduced muscular force, accompanied by gait difficulties and how long the pain and/or reduced force lasted.
We defined clinical effectiveness as an increase of at least 5° in the joint angle, with the joint being passively mobilized at rapid speed. The following measurements were used:
- •
abduction, knees extended, for adductor muscles (long slender adductors);
- •
the popliteal angle for the hamstring muscles;
- •
dorsiflexion of the foot, knee extended, for the gastrocnemius muscles;
- •
dorsiflexion of the foot, knee bent, for the soleus muscles.
1.2.3
Injection technique
All injections were performed by the same practitioner, who had several years of injection experience. For each muscle, the dose was injected at a single point, with a 1 mL-syringe and a 22 gage (40 mm) or a 21 gage (50 mm) needle, depending on the child’s body mass and the muscles injected. Depending on the availability of the radiologist, when it was possible, the injections were performed with ultrasound guidance, especially for multi-site injections. In this case, the ultrasound guidance was carried out in the ultrasound room by a senior radiologist using an ACUSON Sequoia 512 ultrasound system (Siemens). Depending the patient’s body mass, the frequency of the ultrasound probe varied between six and 15. When the guidance was only clinical, the correct needle placement was verified by passively mobilizing the muscle. When the needle moved synchronously with the muscle mobilization, the injection was executed, after aspiration to verify that a blood vessel had not been ruptured.
The product used was Botox ® produced by the Allergan Laboratory, in doses between 5 and 6 UI/kg, according to the marketing authorization (AMM). The dilution of 100 units was carried out in 1 to 2 ml of 0.09% physiological serum, respecting a minimal volume of 0.3 mL per injection site.
All the children were given local anesthesia (Emla ® ), applied at least one hour before the injection. Inhalatory anesthesia (Meopa ® , which is an equimolar blend of oxygen and nitrous oxide) was also used at a rate of 6 to 12 L/minute, starting at least three minutes before the injections and continuing until the end of injection. The injection was delayed a few minutes if the first injection was painful.
1.2.4
Statistical test used
The T test was used to compare the two groups. We also used ANOVA for multi-factor comparisons.
1.3
Results
Fifty-four (54) children were given botulinum toxin injections in their lower limbs ( Table 1 ). Of the total number, 56% were injected under ultrasound guidance. Eighty-five percent (85%) of the children were evaluated with the GMFM-88 before and after the injection(s); the remaining 15% had difficulty understanding the instructions, or were opposed to treatment during one of the two procedures. Most of the children receiving injections had already had botulinum toxin injections in their lower limbs, with at least three months between the injections, in conformity with the AMM.
| Clinical guidance | Ultrasound guidance | Total | |
|---|---|---|---|
| n | 24 | 30 | 54 |
| Boys | 13 | 14 | 27 |
| Girls | 11 | 16 | 27 |
| Age (years) | 8.4 (3–16) | 8.7 (3–17) | |
| Injected muscles | |||
| Adductors | 2 | 13 | 15 |
| Hamstrings | 10 | 37 | 47 |
| Gastrocnemius | 22 | 31 | 53 |
| Soleus | 6 | 9 | 15 |
| GMFM | 17 | 29 | 46 |
One undesirable effect was reported (1.8% of our study population): one instance of asthenia in a 3-year-old child, who had received an injection under ultrasound guidance, was noticed by his family and his usual physical therapist; the situation resolved itself spontaneously after 3 weeks.
1.3.1
Analytical evaluation
The overall clinical effectiveness was good in 51% of the children. The ultrasound guidance increased the effectiveness of the botulinum toxin (overall 54% versus 45%) ( p < 0.05), with the contribution of the ultrasound being clearer for the deep muscles (soleus).
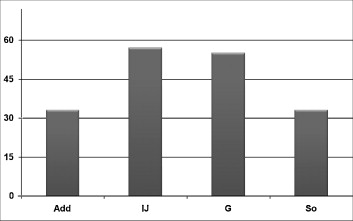
The best clinical effectiveness was found for children under 6 years old (53%) and over 12 (57%). The clinical improvement was better in cases of injections to the hamstring muscle (57%) and/or gastrocnemius muscle (57%) ( p = 0.04) ( Fig. 2 ). This effectiveness also increased with the injected dose: injecting doses over 0.8 UI/kg per muscle was more effective than injecting doses under 0.8 UI/kg per muscle ( p < 0.05).
We did not find any significant difference in clinical effectiveness based on the dilution ( p = 0.17).
1.3.2
Functional evaluation
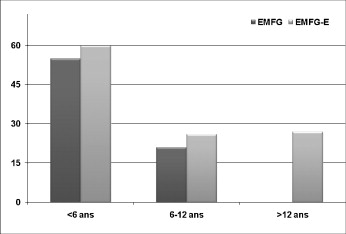
We found an overall functional improvement in 24% of the children. The best results were obtained in the GMFM category E – “Walking, Running, and Jumping”, with a global value of 34% and a value of 55% of functional improvement in the children under 6 years of age ( p < 0.05) ( Fig. 3 ). The functional effectiveness was also improved by using ultrasound guidance ( p < 0.02).
1.4
Discussion
In this study, we found an improved analytical clinical effectiveness in about half of the children. This is less than Mall et al., for whom 14 of 18 patients were clinically improved by injecting botulinum toxin, but the angle of improvement defined in their study is not specified . The same team compared the effectiveness of injections of botulinum toxin in the adductors with a control group injection of a placebo. The effectiveness, as measured by the knee-knee distance at rapid speed, was significantly higher in the group receiving the toxin injections .
For several teams, the effect of toxin injections varies according to the muscular groups injected. Galli et al. found significant clinical improvement, with a gain of around 16° in the gastrocnemius muscles and 6° in the soleus muscles . There was great inter-individual variability in the patients in this study. In a study by Scholtes et al. , after botulinum toxin injections, the patients gained an average of + 5.5° in the triceps surae, + 2.6° in the adductors and + 11.5° in the hamstrings and the rectus femoris . Wissel et al.’s study describes an improvement in the dorsiflexion of the foot of around 11° . Other teams noted no significant modification for passive articular mobilization .
Generally, it seems that the usefulness of the knee-knee distance criterion for evaluating the effectiveness of the toxin injections remains limited if the criterion is measured at low speed, since it measures the passive length of the muscle, on which the botulinum toxin has little to no effect. On the other hand, its use at high speed, as in our study, can be a useful effectiveness criterion if the distance is measured using a goniometer, with the same practitioner taking the measurements to limit the inter-individual variabilities, such as examiner force. It is essential to maintain the sub-calcaneal articulation for the dorsiflexion of the foot during the evaluation, while encouraging the child to relax. In this study, we did not evaluate the evolution of the active joint mobility after injecting the toxin, although certain teams have described this measurement as more sensitive .
Please note that our post-toxin evaluation was performed 3 or 4 weeks before the fitting of successive plaster casts. The decision to apply a cast was sometimes made during this post-toxin evaluation. A new evaluation after the casts were removed would no doubt have shown a clearer analytical improvement.
In this paper, we show that using ultrasound to situate the muscle(s) to be injected allows a significantly greater analytical and functional improvement than was found in the patients injected without ultrasound. This improvement can be easily explained by the fact that the ultrasound allows the tip of the needle to be positioned precisely in the muscle mass, especially when the muscle is a deep muscle. For this reason, we are beginning to inject deep muscles, like the tibialis posterior or upper limb muscles, with ultrasound guidance. In our study, the injecting practitioner already had a lot of experience. It is plausible that the same study, but with a practitioner with less injection experience, would have revealed a more significant difference between the group for which ultrasound guidance was used and the one for which manual guidance was used; the ultrasound would have played a educational role for the doctor performing the injections. Finally, this technique probably provides a better level of injection safety, by permitting the practitioner to see, and thus to avoid, the vascular-nerve bundles.
The best clinical and functional effectiveness was found in children under the age of six, which confirms the results of previous studies . This effectiveness level can be explained by the fact that the fibrotic and retractile phenomena are less significant in young children, thus permitting the anti-spastic effect of the toxin to be revealed more easily. It also seems that motor development is greater during this period, so eliminating the problematic spasticity has a rapid influence on overall motricity.
That this effectiveness is also better in children over 12 than in children from 6 to 12 years old was more surprising. This can probably be explained by the fact that many of the children over 12 had already had musculo-tendinous surgical treatments in their lower limbs, which helped the practitioner target more precisely the muscles to be injected, with higher doses per muscles than those that could be injected with multi-site injections for which the total dose set by the AMM must be distributed among several muscles.
In our study population, as in those described by numerous authors, undesirable effects are quite rare, and don’t seem to be linked to the dose injected . Koman et al. found slight to moderate undesirable effects in 17% of his patients versus 4% when a placebo (physiological serum and human albumin) was injected. These undesirable effects included general or muscular fatigue, falls and unstable gait . In our study, the pain at the moment of the injection was well attenuated by the combination of Emla ® and Meopa ® , and none of our patients complained of significant pain, neither at the time of the injection nor during the control examination. The clinical effectiveness is dose-dependant, with significantly higher analytical improvement with doses greater than 0.8 UI/kg per muscle. Given these two observations, we now tend to use higher doses than the ones described in this article, as do numerous other teams .
The functional improvement measured by GMFM-88 could at first glance seem limited, since it only involved a quarter of our patients. In contrast, Ubhi et al. found a functional improvement in the category “Walking” in 37% of his patients at 12 weeks, but no improvement at 2 and 6 weeks and no improvement in the overall score . We observed that in our population the improvement was clearer in the category, “Walking and Running”. In fact, the other categories were often already saturated at the time of the pre-injection examination in walking children with cerebral palsy, and thus there was no possibility for improvement in these categories. One other factor limiting the pertinence of these functional results is that our post-toxin evaluation was done rapidly, 3 or 4 weeks after the injections, before the successive cast fittings for those who could benefit from this procedure. This time period might be insufficient to reveal a real functional improvement .
1.5
Conclusion
The primary limit of our study is that it was not a double-blind study, which makes interpreting the results less reliable. That our post-toxin evaluation took place before 1 month had passed also probably limits the significance of the results, as does the injection of low doses of the botulinum toxin per muscle for multi-site injections, in order to respect the cumulative dose of 6 UI/kg de Botox ® .
However, this research highlights the usefulness of ultrasound guidance for botulinum toxin injections in the lower limbs of children with cerebral palsy, both on the clinical and functional levels. Because both a radiologist and a re-education specialist need to be available at the same time, the muscle groups that would benefit the most from this technique (e.g., deep muscles? thin muscles?) need to be defined in order to use these specialists’ time more efficiently. This research should be continued with more sensitive methods for evaluating effectiveness and higher doses of botulinum toxin.
2
Version française
2.1
Introduction
Depuis les premières publications de 1993 , la toxine botulinique A dans le traitement de la spasticité des membres inférieurs d’enfants souffrant de paralysie cérébrale est de plus en plus utilisée. L’AMM ne concerne encore actuellement que l’injection des muscles responsables de la déformation dynamique du pied équin, mais la plupart des équipes injecte maintenant de manière habituelle d’autres groupes musculaires que les triceps suraux, avec fréquemment des injections multi-étages . Une évaluation régulière de cette pratique est nécessaire.
L’injection intramusculaire de toxine botulinique se fait généralement avec un repérage clinique . Celui-ci peut cependant être insuffisant pour un bon positionnement de l’aiguille d’injection dans le muscle et ce également sur des muscles superficiels : Chin et al. retrouvent un mauvais positionnement de l’aiguille dans 22 % des gastrocnémiens chez l’enfant . Ce repérage est d’autant plus difficile chez le petit enfant, chez qui les muscles sont parfois très fins. Le repérage par électromyographie ou stimulation électrique améliore la précision de l’injection chez l’adulte, mais son indication chez l’enfant est limitée du fait de la douleur qu’il entraîne et/ou de la coopération parfois difficile des patients . Certaines équipes réalisent les injections sous-anesthésie générale pour limiter ces difficultés lors d’injections multisites ce qui ne peut cependant pas être réalisé de manière régulière et sans risque chez ces enfants qui nécessiteront souvent de nombreuses séances d’injections durant leur croissance.
Le repérage échographique semblent être une bonne alternative chez l’enfant atteint de paralysie cérébrale : il permet d’identifier le muscle à injecter et de vérifier la position de l’extrémité de l’aiguille au sein du corps musculaire défini ( Fig. 1 ).