Fig. 3.1
Sensory innervation of the face via branches of the three major divisions of the trigeminal nerve. V1 ophthalmic division, V2 maxillary division, V3 mandibular division
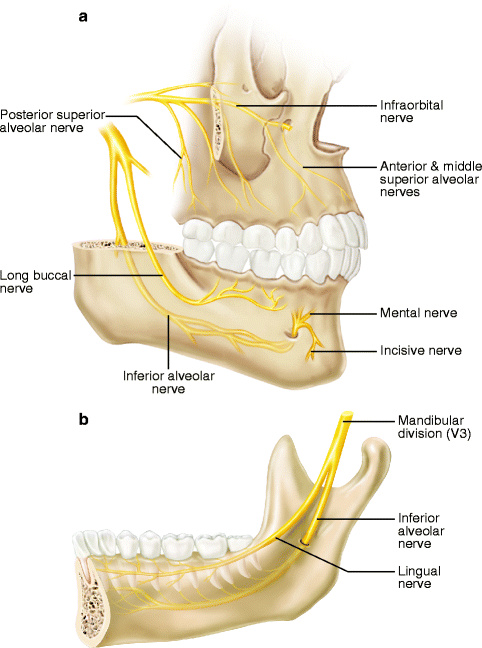
Fig. 3.2
Important sensory branches of the trigeminal nerve in the oral cavity: (a) labio-buccal aspect of maxilla and mandible; (b) lingual area of mandible
Rarely do injuries occur to the other branches of the TN5, such as the anterior, middle, and posterior superior alveolar, nasopalatine, and greater palatine nerves of V2 and the mylohyoid, auriculotemporal, and incisive nerves of V3, perhaps because alteration of sensation in the affected areas is not readily perceived by patients and does not seriously interfere with orofacial functions, or merely that the paresthesia resolves rapidly [76]. For example, temporary, but sometimes prolonged, numbness of the palate is common after a LeFort I maxillary osteotomy because of involvement of the nasopalatine and greater palatine nerves. However, it is seldom a long-term patient complaint and does not seem to interfere with speech, mastication, or drinking or swallowing liquids [69]. The buccal and labial gingivae are routinely anesthetic following a LeFort I osteotomy because the terminal fibers of the middle and anterior superior alveolar nerves are severed by the usual circumvestibular incision. Recovery of this sensation occurs within a few weeks or months, and the interval of gingival insensitivity has little or no effect on oral function. The mylohyoid nerve which branches from the IAN in the pterygomandibular fossa provides motor innervation to the mylohyoid muscle and the anterior belly of the digastric muscle. In some patients, it has a sensory component that supplies a small area of skin in the submental area where loss of sensation is not often perceived by the patient. Likewise, the auriculotemporal nerve (ATN) is frequently injured during temporomandibular joint surgery, parotid gland surgery, or rhytidectomy, but the alteration of sensation in the periauricular region generally resolves within a few months and is seldom a problem for the patient. Occasionally, however, injury to the ATN is associated with the development of Frey’s syndrome (gustatory sweating, see Sect. 3.5.7) that can be a significant aggravation for the afflicted patient [132]. Also, the incisive nerve is often intentionally sectioned to allow for maximal lateralization or advancement of the IAN during nerve repair surgery after injury or to allow for lateral repositioning for dental implant placement. The resulting loss of sensation in the mandibular labial gingiva and anterior teeth does not present a problem for most patients, although the lack of tactile proprioception in the incisors may be frustrating for some patients. In addition, however, an amputation neuroma may develop rarely on the proximal stump of a transected incisive nerve possibly leading to painful neuropathies [14].
3.2.1 Supraorbital and Supratrochlear Nerves
The supraorbital nerve (SON) traverses along the superior orbital fissure above the bony orbit and exits through the supraorbital foramen, or notch, in the superior orbital rim of the frontal bone. From this point, the SON and its branches proceed medially, laterally, and cephalad to supply sensation to the eyebrow, forehead, and anterior scalp. The SON has a “superficial” (lateral) division and a “deep” (medial) division. The superficial division courses superficially over the frontalis muscle and supplies sensation to the skin of the forehead, while the deep division proceeds more cephalad beneath the galea aponeurotica to innervate the frontoparietal region of the scalp [65]. This deep (medial) division has implications in the surgical dissection utilized for a forehead or brow-lift procedure (see Sect. 3.5.9). The supratrochlear nerve (STN) exits from beneath the superior orbital rim about 1 cm medial to the supraorbital foramen and provides branches to the upper eyelid and lower midportion of the forehead. The patient seldom notices the loss of sensation from the STN alone following forehead injury or surgical procedures.
3.2.2 Infraorbital Nerve
The infraorbital nerve (IFN), the most important branch of V2, traverses the inferior orbital canal below the floor of the orbit and exits via the infraorbital foramen inferior to the inferior orbital rim. From there it divides into several branches as it proceeds peripherally. Its locations within the inferior orbital canal and following its exit from bone make it susceptible to injury from trauma or various surgical procedures. The injured IFN may produce symptomatic neurosensory dysfunction in the upper lip and middle third of the face (see Sects. 3.5.3 and 3.5.4).
3.2.3 Inferior Alveolar Nerve
The inferior alveolar nerve (IAN) leaves V3 in the pterygomandibular space and courses anterolaterally to the medial surface of the mandible into which it enters at the mandibular foramen. From here, its location within the inferior alveolar canal (IAC) can be highly variable, superoinferiorly, between the molar and premolar teeth and the mandibular inferior border and, mediolaterally, between the lateral and medial mandibular cortices (Fig. 3.3). Recognition of this variability of position of the IAN is important in planning a surgical procedure for the removal of mandibular third molars (M3s), correction of mandibular developmental deformities with orthognathic surgery, repair of mandibular fractures, placement of dental implants, and endodontic periapical surgery (see Sects. 3.5.2, 3.5.3, 3.5.4, 3.5.5, and 3.5.7). This location can usually be determined from plain radiographs in most patients [39, 56]; however, in those patients who are suspected of having an intimate relationship between the IAN and an approximating tooth, implant, or other object or structure (based upon plain-film assessment), the availability of newer imaging techniques (computed tomography (CT), cone-beam computed tomography (CBCT)) has made possible the precise and accurate determination of the position of the IAN within the mandible (see Chaps. 5 and 11).
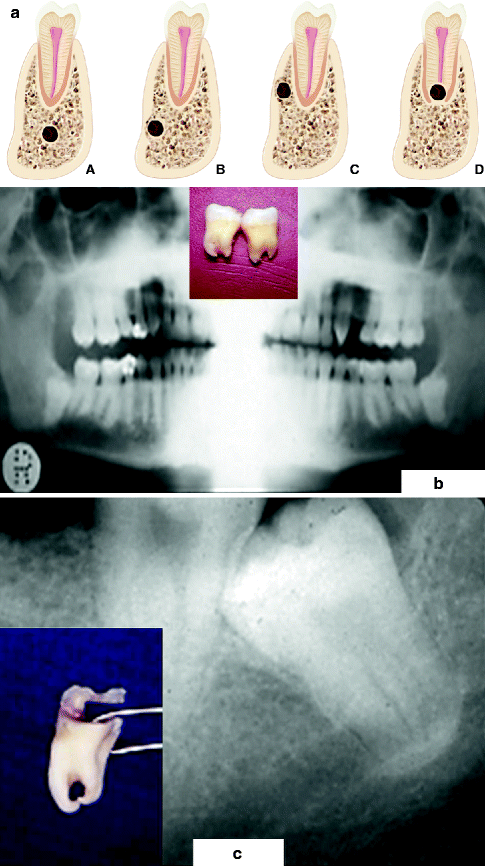

Fig. 3.3
Variable locations (left, a) of the inferior alveolar canal (IAC) in the mandible molar region (seen in cross section) which can be determined from preoperative imaging studies: (A) the IAC lies several millimeters inferior to the tooth root apex, a favorable position during M3 removal; (B) the IAC, situated inferiorly, is grooving the lateral cortical bone, placing it at risk during the mandibular sagittal split ramus osteotomy (MSSRO); (C) the IAC, located superiorly, again is grooving the lateral cortical bone, placing it at risk when performing the MSSRO or inserting superior border monocortical internal fixation screws; (D) the IAC lies within a groove in the root apex, posing a risk of injury during the removal of the tooth. Bilateral impacted mandibular third molars (M3) in which the roots are straddling the IAC (middle, b). An M3 whose roots were perforated by the IAC (right, c). The IAN was severed during M3 removal and was later successfully repaired with microsurgery
3.2.4 Mental Nerve
The mental nerve (MN) arises from the IAN in the inferior alveolar canal in the premolar region. The MN then courses superiorly and posteriorly to exit the lateral surface of the mandible through the mental foramen (MFN), generally located between and slightly inferior to the apices of the mandibular first and second premolar roots. Vertical or horizontal incisions and submucosal dissections in the mandibular buccal vestibule should be performed with great caution in this area. The level of exit of the MN is generally several millimeters superior to the level of the inferior alveolar canal, a relationship that impacts upon the placement of a horizontal osteotomy for mandibular symphysis repositioning or genioplasty (see Sect. 3.5.9). As it exits the MFN, the MN usually divides into three distinct branches that pass inferior, lateral, and anterior (lower labial branches, LLBs) to supply the lower labial mucosa and skin of the lower lip. Occasionally, there is an anatomic variation in which the MN exits the mandible as two separate branches via two bony mental foramina (Fig. 3.4). Knowledge of the position of the LLBs of the MN [1] aids the clinician in determining appropriate incision designs in the lower labial mucosa for various procedures (such as biopsy of minor salivary glands, excision of submucosal masses, and mandibular symphysis procedures) while minimizing the risk of injury to the MN. In general, the LLBs of the MN proceed in an anteromedial direction at an angle of about 36 % to the horizontal plane of the lower lip, so an incision in the lower labial mucosa for removal of a submucosal mass should parallel the direction of these branches. A U-shaped incision with its lateral aspects parallel to the LLBs should be made to expose the mandibular symphysis (Fig. 3.5). When the patient has lost posterior mandibular teeth and there is alveolar bone atrophy in the mandibular body region, the MFN and/or the IAC may be located at, or near, the alveolar crest, placing the MN or the IAN at risk from incisions or other surgical manipulations in this area [55, 77, 78] (Fig. 3.6).
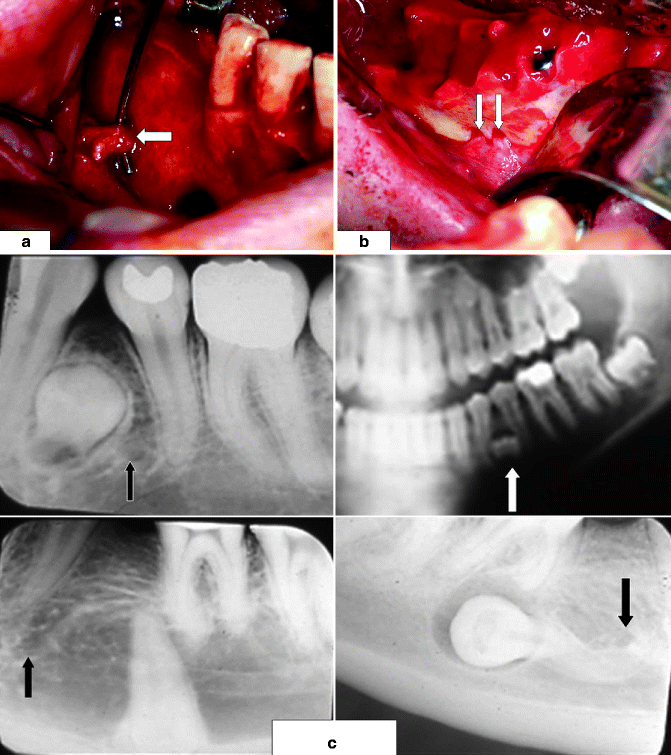
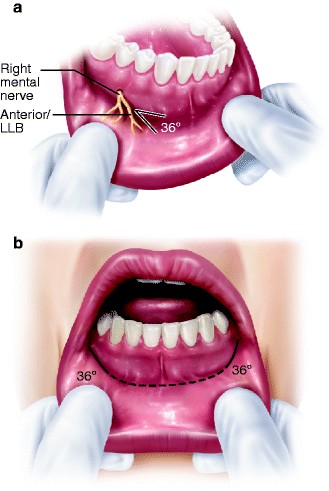
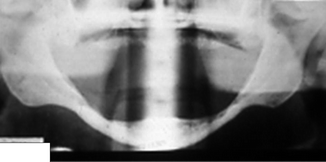

Fig. 3.4
Mental nerve (MN, indicated by white arrow) usually (a) exits the buccal surface of the mandible inferior to the root apices of the two premolar teeth; (b) a view of patient with two right mental foramina, each with a MN; (c) radiographic views of impacted mandibular premolar teeth, each of which is in close proximity to its adjacent mental foramen (arrows), posing a risk of MN injury during their removal

Fig. 3.5
(a) MN gives off its anterior/lower labial branches (LLB) which course anteriorly at an angle of about 36° with the horizontal plane of the lower lip. An incision in this area should parallel the LLB; (b) a labial vestibular incision for access to the mandibular symphysis has its lateral wings (solid black lines) parallel to the LLB. Remainder of the incision is a dotted line

Fig. 3.6
Severe atrophy of the mandible. IAC and MF are at or near the crest of the residual alveolar ridge
3.2.5 Lingual Nerve
The lingual nerve (LN), after it leaves V3 in the pterygomandibular space, proceeds anteriorly where it assumes a variable relationship to the medial surface of the mandible in the third molar (M3) area. Cadaveric dissections and clinical experience have shown that the LN in the M3 area may be located in intimate contact with the medial mandibular periosteum at, or above, the level of lingual crest of bone (Fig. 3.7) or one to several millimeters below the alveolar crest at various distances (from 0 to several millimeters) medial to the lingual mandibular periosteum [20, 63, 86]. It has been noted that these nerve-bone relationships may not necessarily change in patients who subsequently lose their teeth and undergo mandibular atrophy [55]. The position of the LN on one side of a bilateral cadaver dissection [103] and as seen in clinical experience is not a reliable predictor of its position on the contralateral side. The frequently noted intimacy of the LN and the mandible in the third molar region increases the risk of LN injury from removal of M3s or other surgical procedures in the retromolar pad area (see Sects. 3.5.2, 3.5.3, 3.5.4, and 3.5.5). As the LN courses anteriorly from the M3 region, it again may assume a variable relationship with the submandibular salivary duct and the submandibular salivary gland. In some patients, the LN runs medially inferior to the submandibular duct and then into the floor of the mouth and tongue musculature. In other patients, the LN runs through or inferior to the submandibular gland to reach the body of the tongue muscle [88, 89]. In these latter two relationships, the LN might be in jeopardy during surgical procedures of the sublingual salivary gland, submandibular gland, or Wharton’s duct. As the LN proceeds anteriorly from the M3 area and into the floor of the mouth, it assumes a more tortuous course. This has implications for the surgical repair of LN injuries in that dissection and mobilization of the distal portion of a severed nerve often allows it to be advanced without tension to approximation with the proximal nerve stump. The nerve gap is eliminated and a direct neurorrhaphy, rather than an indirect reconstruction with a nerve graft or conduit, may be performed [12, 13].
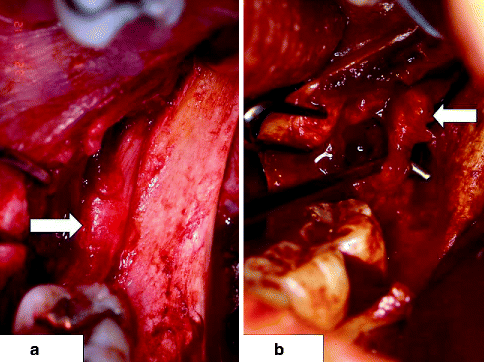

Fig. 3.7
(a) Intact left lingual nerve (LN, arrow), exposed during a mandibular ramus surgical procedure, is located at level of alveolar crest in mandibular retromolar area; (b) left LN injured during mandibular third molar removal several months previously has developed a neuroma-in-continuity (arrow)
3.2.6 Long Buccal Nerve
The long buccal nerve (LBN) leaves V3 in the pterygomandibular space and crosses lateroinferiorly in a supraperiosteal location over the deepest concavity of the external oblique ridge of the mandibular ramus, or up to 12 mm inferior to this point. There the LBN may separate into several smaller branches or continue as a single structure into the mandibular buccal vestibule in the molar area where it then sends multiple smaller branches medially, laterally, and anteriorly to supply the buccal molar gingiva, buccal mucosa, and mandibular vestibule, respectively [52]. While the main trunk of the LBN as it crosses the external oblique ridge is often 1 mm in diameter, it is seldom noted in surgical dissections in the retromolar pad or vestibule of the posterior mandible unless it is the subject of exploration and repair. When the LBN crosses below the greatest concavity of the external oblique ridge, it may be at risk of injury from incisions in the posterior mandibular buccal vestibule, such as those performed for M3 removal, mandibular ramus osteotomies, or open reduction of posterior mandibular body, angle, ramus, or condylar fractures. In the majority of patients, transection of the main trunk of the LBN, or one or more of its branches, is associated with little, if any, perceived sensory aberration [76], possibly due to a high mechanosensory threshold of this nerve [51]. However, in some patients, a LBN injury results in significant sensory dysfunction, especially if a painful neuroma develops on the proximal stump of a severed LBN [11] (Fig. 3.8).
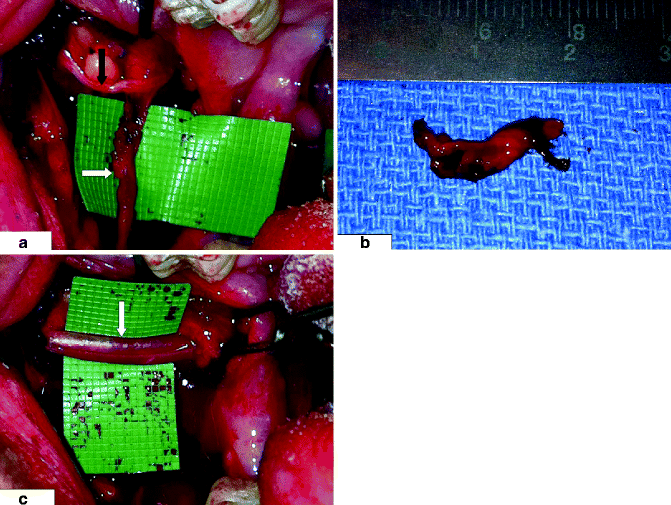

Fig. 3.8
Right long buccal nerve (LBN) is shown, (a) with its normal-appearing main branch (black arrow) traversing laterally into the cheek mucosa and an abnormal anterior branch with neuroma (indicated by white arrow). The patient developed stimulus-evoked pain in the right buccal vestibule following removal of the right third molar tooth. The pain resolved (b) after resection of the anterior branch and its neuroma; (c) main branch of the LBN is surrounded by membrane sheath (indicated by white arrow) to facilitate healing after resection of anterior branch
3.3 Types of Nerve Injury
3.3.1 Clinical Categories of Nerve Injury
Clinically, peripheral nerve injuries are divided into two categories: closed and open injuries. The vast majority of TN5 injuries occurring during elective surgery, except those nerve resections which are planned as part of ablative surgery, are unobserved or are unsuspected by the surgeon at the time of operation [110]. Only in retrospect, when the patient returns with a complaint of sensory dysfunction, is the diagnosis established and the surgeon obliged to evaluate the situation further. Such an injury, not directly observed by the surgeon at the time of its occurrence, is termed a closed (or unobserved) injury. When a nerve injury is noticed at the time of surgery, whether it is produced intentionally, such as during surgical excision of a malignant tumor in which the nerve is involved, or unintentionally, such as during an elective, non-ablative operation, this is called an open (or observed) injury. An open injury is documented in the surgeon’s notes or operative report, and if the nerve is not to be repaired at the time it occurs, the injured area of the nerve may be tagged with fine, nonabsorbable, nonreactive sutures (such as 8-0 monofilament nylon) to assist the surgeon who does the subsequent microsurgical repair in identifying the proximal and distal nerve stumps.
3.3.2 Mechanisms of Nerve Injury
There are many aspects of surgical manipulation that can lead to TN5 injury (Table 3.1). Some of these might be recognized clinically, if the nerve is exposed, and repaired at that time or within a short time after injury (as in a delayed primary repair at 3 weeks) [99]. However, within several weeks, the healing process has begun and scar tissue has formed, and although these events may render the surgical repair technically less difficult [79], they alter the appearance of the injured nerve, and frequently make the mechanism of injury difficult to determine clinically or histologically. From direct clinical observation and other considerations [97], it has been proposed that the TN5 may be injured via the following: (1) sharp incision (as from a scalpel or an anesthetic needle) that may cause a partial (one or more fascicles) or total (all fascicles) nerve transection; (2) blunt trauma associated with maxillofacial injuries or from instrumentation such as elevation of a mucoperiosteal flap; (3) stretching, compression, or laceration from displaced bone fragments in facial bone fractures; (4) manipulations during reduction of fractured bone fragments or osteotomized bone segments that produce nerve compression or crushing; (5) a high-speed rotating bur during bone removal or a slower-speed drill preparing dental implant sites or bone holes for internal fixation screws that causes ragged and irregular nerve shredding; (6) impaling the nerve with an internal fixation screw; (7) prolonged or excessive retraction of the nerve that induces ischemia and a stretching (or neurapraxic) injury; or (8) contact with a toxic root canal medicament or sealer or other chemical medications (such as tetracycline placed into a tooth-extraction socket) that generate a chemical burn of the nerve.
Table 3.1
Etiology of TN5 injuries
Procedure | Nerves affected | Mechanism of injury |
|---|---|---|
Local anesthetic injection | IAN, LN | Direct needle trauma |
Toxic effect of anesthetic | ||
Bleeding, hematoma | ||
M3 removal | IAN, LN, LBN | Incision |
Flap retraction | ||
Rotating bur, osteotome | ||
Compression (bone, root) | ||
Suturing | ||
Socket medication | ||
Orthognathic surgery: | IFN, IAN, LN | Drill, osteotome, saw |
Lefort I, MSSRO, MIVRO | Internal fixation | |
Nerve retraction | ||
Nerve compression | ||
Maxillofacial trauma: | SON, IFN, IAN, MN | Compression |
Fracture, laceration, GSW | Severance | |
Avulsion | ||
Internal fixation | ||
Preprosthetic surgery: | IAN, LN, MN | Chemical burn |
Ridge augmentation | Compression, suture | |
Vestibuloplasty | Compartment syndrome | |
Dental implants | Rotating bur | |
Endodontic treatment: | IAN, MN | Overinstrumentation |
Root canal filling | Compression | |
Periapical surgery | Chemical burn | |
Salivary gland surgery: | LN | Dissection |
Submandibular, sublingual | ||
Ablative surgery: | IAN, MN, LN | Unintentional injury |
Benign cysts/tumors | Intentional nerve resection | |
Malignant tumors | ||
Cosmetic facial surgery: | SON, MN, ATN | Dissection |
Genioplasty, facelift, forehead/brow lift | Compression | |
Rotating bur, saw |
3.4 Incidence of Nerve Injuries
Reliable statistics about the frequency of TN5 injuries are hard to obtain since so much of the activity (dental treatment, intraoral surgery, and cosmetic procedures) associated with these injuries is performed in private practice offices where either thorough documentation is incomplete or the databases lack the capability for retrieval of pertinent information on nerve-injured patients. Even in hospitals, recognition of the event of a nerve injury may not take place until after the patient has been discharged, rendering a retrospective database search futile or misleading in many cases, even with the most sophisticated electronic medical record computer systems; without the data input, there can be no data retrieval. In the absence of national or international registries for the accumulation of nerve injury data, most of the current information concerning the causes and frequency of TN5 injuries has come from group surveys, reports of individual experience in the performance of certain procedures, or retrospective or prospective case reports or case series of the results of microsurgical repair of TN5 injuries in the literature. There is little doubt that the incidence of TN5 injuries from all etiologies, but especially those resulting from local anesthetic injections, is underreported [100]. This information is summarized in Table 3.2, and it is discussed further below in relation to the individual causes or mechanisms of TN5 injuries.
Table 3.2
Incidence of TN5 injury based on procedure
Procedure | Posttraumatic NSDa (%) | Postoperative NSDb (%) | Permanent NSDc (%)d |
|---|---|---|---|
Local anesthetic injection | N/Ae | 0.0033–3.3 | 0.54 |
M3 removal | N/Ae | 0.10–0.40 | 0.001–0.040 |
Genioplasty | N/Ae | 100 | 3.33–10.0 |
Mandibular SSRO | N/Ae | 63.3–83.0 | 12.8–39.0 |
SSRO + genioplasty | N/Ae | 100 | 66.6 |
Mandibular IVRO | N/Ae | 18.0 | 0.01 |
Mandibular DO | N/Ae | 46.7 | <5.0 |
Mandible fracture | 46.0–58.5 | 76.1–91.3 | 38.8 |
ZMC fracture | 52.0–100 | 7.7–55.0 | 37.0 |
Mandibular vestibuloplasty | N/Ae | 100 | 50–100 |
Dental implant | N/Ae | 1.7–43.5 | 0–15 |
Perhaps of equal importance to the “clinical outcome” of peripheral TN5 injuries is the patient’s perception of his or her neurosensory status and their ability to carry out the usual oral and facial functions that depend upon sensory input. Some patients who achieve a level of “functional sensory recovery” based upon clinical testing may still continue to experience adverse symptoms or interference with function and activities of daily living, while others will tolerate compromised oral or facial sensation without significant difficulty. In general, however, most patients who experience greater neurosensory improvement after surgical repair of TN5 injuries report lower frequencies of related oral/facial dysfunction [119]. Assessment of the degree of recovery of sensory function and its long-term effects on quality of life (i.e., “patient-centered research”) deserve more attention of clinicians and researchers, since the care of the nerve-injured patient [68, 105] and, indeed, all types of patients [16] will continue to evolve in the future.
3.5 Causes of Nerve Injury
3.5.1 Local Anesthetic Injections
Injection of local anesthetics for dental treatment or oral and maxillofacial surgery is by far the procedure most frequently performed in proximity to peripheral branches of the TN5. It is estimated that the average general dental practitioner administers between 3 and 10 mandibular nerve blocks per day, or 20–25 per week, and he/she sees some type of IAN or LN involvement (either paresthesia at the time of the injection and/or subsequent sensory dysfunction) as a result of the injection about once every 2–8 weeks. This data would imply an incidence of nerve injury of between 1:30 (3.3 %) and 1:300 (0.003 %) [47, 100] (Table 3.2). Given that it is essentially a blind (albeit trained and practiced) maneuver within the pterygomandibular space, it seems curious that the incidence of injection-associated IAN and LN injuries is not greater. Is this a case of underreporting or a testament to the skill of the average dentist or merely luck? Of course, the goal of the injection is to deposit the anesthetic solution in close proximity to the nerves being anesthetized and to avoid actual contact with the nerve. If this is achieved and apparently it is in the vast majority of injections, then the happenstance of needle contact with the nerve and the possible sudden dysesthesia (“electric shock,” the possibility of which is always a patient’s fear) is avoided in most cases, or it may be that contact between the needle and the nerve may not result in any significant neurosensory dysfunction. The dysesthesia resulting from needle contact with the nerve is not a reliable indicator of subsequent significant, prolonged or permanent, sensory dysfunction, however. “Needle shock” does not always occur in patients who subsequently fail to regain sensation in the usual time frame, and in many patients who experience the sudden pain of needle to nerve contact, there is no subsequent sensory dysfunction [100]. In those patients who are under intravenous sedation or general anesthesia before the injection of local anesthetic is performed, there will be no recollection of needle contact with the nerve [82].
There are three proposed mechanisms of nerve injury resulting from a local anesthetic injection [101]. These include the following: (1) direct trauma, the needle may pierce the nerve, injuring one or more fascicles, and (2) chemical toxicity, the anesthetic solution may have a neurotoxic effect. All local anesthetic solutions have to meet FDA specifications and are thought to be nontoxic in the concentrations used to produce local anesthesia in human patients. Recently, however, mention has been made of the potential toxicity of a 4 % solution of articaine hydrochloride when used for local anesthetic nerve blocks for dental procedures [54] (see Chap. 5 on Injection Injuries). Also, there is the possibility that a cartridge containing any of the commonly used local anesthetics (i.e., lidocaine, mepivacaine, bupivacaine) could have a leak, and when placed into storage in a sterilizing solution (alcohol or other chemical that is neurotoxic), that cartridge might become contaminated. Upon injection of the contents of the cartridge to produce local anesthesia, the toxic sterilizing solution could be carried into contact with the nerve. The use of a disclosing agent (such as methylene blue) in the sterilizing solutions where anesthetic cartridges are stored in professional offices and clinics could eliminate this iatrogenic nerve injury; (3) bleeding and hematoma formation: the injection needle pierces or tears a blood vessel in the mesoneurium or epineurium of the nerve, causing localized bleeding and formation of a hematoma around or within the internal structure of the nerve thereby producing a compression effect on the nerve. In some patients, the hematoma is rapidly resorbed, and any effect on sensory function is transient. In others, the hematoma organizes and is replaced by scar tissue that exerts a continued compression on the nerve, and neurosensory dysfunction persists. Which specific one of these effects, a combination of several effects, or other mechanisms as yet unknown occurs in a given patient remains an unresolved question at this time [100].
Following a protocol for the administration and documentation of local anesthetic injections might minimize the risk of nerve injury and provide an impetus for proper follow-up evaluation, which increases the likelihood that a nerve injury is recognized and that rapport with the patient is maintained [82]. When the patient is fully conscious, the clinician proceeds to insert the local anesthetic needle into the proper location (i.e., pterygomandibular space). In the absence of the patient’s complaint of sudden pain or shocking sensation (dysesthesia, which may radiate to the lower teeth, lower lip, mandible, or tongue), the syringe is aspirated. If the aspirate is free of blood, the local anesthetic is administered with the needle position unchanged. If there is a bloody aspirate, the needle is withdrawn 2–3 mm and aspiration is repeated. If the aspirate is then clear, the local anesthetic is injected with the needle in the new position. If the patient complains of sudden pain or shocking sensation, the needle is withdrawn 2–3 mm. Following a clear aspiration, the anesthetic is injected in this new position. If there is either a bloody aspirate or a dysesthesia associated with the injection, the incident is noted in the patient’s record, and a follow-up evaluation of sensory function is done at the patient’s next visit (see Chap. 10). When the patient is under general anesthesia or intravenous sedation, the patient will not be able to react to a dysesthesia. Therefore, aspirate before injecting and proceed as described above.
While the IAN and the LN are the TN5 nerves most frequently injured by local anesthetic injections [101, 102], injuries to other branches including the LBN, nasopalatine, mental, and IFN have been seen by the authors.
For further discussion of this topic, the reader is referred to Chap. 4.
3.5.2 Mandibular Third Molar Removal
Removal of third molar teeth is the most frequently performed surgical procedure in oral surgery practice [95]. It has been estimated that some oral and maxillofacial surgeons (OMFS) remove as many as 25 or more M3s per week in their office practices. During the latter half of the twentieth century, a number of reports (from Europe, the United Kingdom, New Zealand, and the United States) indicated that an injury to the IAN or LN during M3 removal occurred in 1.0–6.0 % of patients, with 0.1–1.0 % of these injuries failing to resolve within a few months and becoming permanent in the absence of surgical intervention [3 23, 24, 27, 46, 53, 64, 129].
More recently, a prospective study conducted by the American Association of Oral and Maxillofacial Surgeons (AAOMS) of a selected group of 63 American oral and maxillofacial surgeons who removed 8,333 M3s from 3,760 patients over a 1-year period (January–December 2001) found an incidence of IAN injury of 1.1 % on the left side versus 1.7 % on the right side, while the LN was involved in 0.3 % (equal on both sides). These figures were for the immediate postoperative period only, so there was no indication of whether any of these injuries failed to resolve spontaneously [49]. A retrospective survey of California OMFS showed that in 95 % of practices surveyed (n = 535), over a 1-year period, 94.5 % experienced one or more IAN injuries, and 53 % had one or more LN injuries. Over their practice lifetimes, 78 % of these OMFS reported one or more cases of “permanent” IAN injury, while 46 % indicated one or more instances of “permanent” LN injury. The mean rate for any IAN involvement (temporary or prolonged) was 4/1,000 (0.4 %), and the permanent IAN injury mean rate was 0.4/1,000 (0.04 %). For the LN, the mean rate for any involvement was 1/1,000 (0.1 %), while that of permanent LN injury was 0.1/10,000 (0.01 %). In most cases of IAN injury, the surgeon was aware of the cause of the injury, probably due to the surgeon’s knowledge of the relationship of the M3 to the inferior alveolar canal as seen on the preoperative panoramic radiograph. However, in most LN injuries, the surgeon did not know the cause, which may be because the LN was not imaged preoperatively and not directly visualized during the procedure. Nerve injury rates varied inversely with the numbers of M3s removed per year by each surgeon and his/her total years of surgical practice, emphasizing the importance of experience in the reduction of M3 surgical complications [110].
Removal of an impacted mandibular third molar (M3) presents unique surgical requirements, especially with regard to avoidance of nerve injuries. Even in an operation that is conducted according to the existing standards of care by a well-trained and experienced OMFS, it is accepted and expected that complications may occur. Mechanisms of TN5 injury while removing M3s can occur during local anesthetic injection (see above), incision placement, soft tissue flap retraction, removal of bone, sectioning of teeth, elevation of teeth, suturing, and placement of socket medications. Delayed injury of the IAN may occur when the IAC is disrupted during M3 root elevation or removal [25]. During the osseous healing process, bone proliferation may have the effect of narrowing the diameter of the IAC and compressing the IAN, a “closed box” effect similar to the sequelae of increased intracranial pressure on the intracranial contents as a result of a closed head injury. Discussed below are suggestions for minimizing the risk of TN5 nerve injury during the removal of M3s.
Imaging studies are indispensible in the preoperative planning for M3 removal. An acceptable radiograph displays the entire tooth, the surrounding alveolar bone, the periapical area, and the inferior alveolar canal (IAC). A plain panoramic view is most often the basic imaging study for M3 evaluation. Although the depth of the tooth within the mandible (soft tissue, partial bone, or complete bone impaction) and the angulation of the tooth (vertical, horizontal, mesioangular, distoangular) are certainly important to the surgeon, perhaps most critical to the prevention of IAN injury is the relationship of the M3 roots to the IAC [56]. Several conditions seen on a plain films may indicate the likelihood of exposure of the IAN during M3 removal including (1) darkening (decreased radiodensity) of the tooth root where it is crossed by the IAC, (2) narrowing of the IAC where it crosses the M3 root, (3) interruption of the white lines (cortical walls) of the IAC, (4) diversion of the IAC, and (5) narrowing of the M3 roots [115]. When a plain radiograph suggests a possible intimate relationship between an M3 and the IAC, this situation may be clarified with advanced radiographic technology [118]. Computed tomography (CT) provides a three-dimensional view of soft tissue and bony anatomy. Although the CT scan was available only in the hospital setting, the introduction (in the 1990s) of cone-beam computed tomography (CBCT) brought this important imaging technology to office surgical practice. In the evaluation and treatment planning for M3 removal, CBCT is invaluable in determining the relationship of M3 roots to the IAC [109]. For more information on this topic, see Chap. 5.
The location of the soft tissue incision is important in avoiding injury to the LN. The posterolateral extension of the buccal incision from the mesiobuccal corner of the mandibular second molar often encounters the LBN, but injury to this nerve is only rarely symptomatic. Far more important is that the incision is not carried directly posteriorly or even posteromedially where it may cross the path of the LN, which may be located in the soft tissues overlying the impacted M3 [63].
Soft tissue flap retraction, while allowing access and visualization of the operative site, also provides protection to important neighboring structures such as the LN. Lingual flap retraction, a mainstay of the split-bone technique for M3 removal [111], might be followed by a temporary paresthesia due to mild compression of the LN, but the incidence of permanent paresthesia is not increased [94]. The LN retracting instrument protects the nerve from more severe, possibly permanent, injury in case an errant osteotome, elevator, or high-speed rotating bur penetrates the lingual cortical bone [43].
Removing soft tissue pathology from around the crown of an M3 (e.g., granulation tissue, enlarged follicular sac, dentigerous cyst) should be performed with care. If the lingual bone has been eroded or perforated, the pathologic tissue, mandibular lingual periosteum, and LN may be adherent to one another and inadvertently removed en masse, causing an avulsion injury to the LN. Periapical pathology may be located adjacent to the IAC, and curettage of the socket should be performed gently to avoid encroachment on the IAC.
During removal of bone or sectioning of the tooth, great care is taken regarding the positions of the LN and the IAN [56, 76]. Placement of a lingual retractor (see above) protects the LN if it is necessary to remove lingual bone with the high-speed drill or osteotomes in order to expose, section, or deliver the M3 [108]. When sectioning the tooth with the high-speed drill, the rotating bur should section only three-fourths of the way through the M3, thus avoiding direct trauma to an adjacent LN or IAN. Completion of the separation of the tooth fragments is performed with an elevator. Vectors of force created when elevating teeth should be appreciated; for example, upward and posterior elevation of the crown of a mesioangular M3 may cause a reciprocal anteroinferior rotation of the root apex and possibly adjacent bone into the IAC, causing compression of the IAN, and this would be a situation that would undoubtedly go unnoticed during the procedure. Application of excessive force during tooth elevation, especially in a patient with extensive bone resorption, or where a large amount of bone has been removed to expose the tooth, may cause a fracture of the mandible, and fracture displacement may cause significant IAN injury.
Partial odontectomy [40] or coronectomy [104] can be considered as an alternative treatment to M3 removal in certain instances, including when the roots of an M3 reside in close approximation to the IAC, when there is an atrophic mandible containing a deeply impacted M3 and there is risk of pathologic fracture of the mandible, and if cases of advanced patient age. After the crown of the tooth is removed, the roots are left in situ. Subsequent development of infection or other complications such as root migration or even IAN paresthesia may occur rarely. The root migration in an occlusal direction in some patients away from the IAC may allow their subsequent removal with less chance of IAN involvement.
In general, if either the LN or the IAC contents were directly visualized during M3 removal, it is not advisable to medicate the socket with antibiotics (cones, powder, etc.) at the conclusion of the operation or to place analgesic liquids or pastes into the socket afflicted with alveolar osteitis several days following the extraction. If such substances (e.g., eugenol, tetracycline, Surgicel) come into direct contact with the LN or IAN, they have the potential to cause a chemical burn with long-term paresthesia, including unpleasant dysesthesia [33].
When lingual bone in the M3 area has been eroded by pathology, fractured off during removal of an ankylosed tooth, or removed surgically with a bur or osteotome, the LN may be exposed and vulnerable during suturing of the lingual soft tissue flap. This may cause a compressive injury to the LN, but long-term paresthesia is unlikely via this mechanism of injury.
For further discussion, the reader is referred to Chap. 5.
3.5.3 Orthognathic Surgery
The most common surgical procedures to correct developmental facial deformities associated with dental malocclusions in the upper jaw are the LeFort osteotomies (LeFort I, or horizontal maxillary osteotomy; Lefort II, or pyramidal osteotomy; and LeFort III, or transverse facial osteotomy) and, in the lower jaw, the mandibular sagittal split ramus osteotomy (MSSRO), the mandibular intraoral vertical ramus osteotomy (MIVRO), and mandibular distraction osteogenesis (MDO). Of these, the LeFort I and MSSRO pose the greatest risk of significant TN5 injury (to the IFN in the maxilla and to the IAN in the mandible) [38].
Injury to the IAN during MSSRO has been studied extensively [32], and it is well known that sensory dysfunction of the IAN following MSSRO is nearly universal (∼100 %) among patients in the immediate postoperative period, being reported in 63.3–83.0 % of patients. When patients are followed for more than 1 year, the incidence of prolonged or permanent IAN injury varies from 12.8 to 39.0 %. In both the immediate postoperative evaluation and in the longer follow-up periods, both objective and subjective methods of sensory assessment were used; however, following MSSRO many patients are satisfied with their neurological status and do not request further treatment for residual IAN sensory dysfunction [41, 133]. See Table 3.2. Factors which have been found to increase the risk of IAN injury during MRSSO include the position of the IAC [131], especially when it is located just medial to or within the lateral cortical plate of the mandible [130, 134]; patient age, especially greater than 40 years [2, 5]; type of fixation, whether wire osteosynthesis or mono- or bicortical screws [42, 70, 80]; magnitude of mandibular advancement, and whether or not there was manipulation of the IAN [133], whether an additional osteotomy (such as for genioplasty) was performed [124], and also the duration of the operation [120].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








