Epstein-Barr Virus Infection in Children
Katherine Luzuriaga
John L. Sullivan
In 1964, Anthony Epstein and Yvonne Barr isolated a new herpesvirus from Burkitt lymphoma tumor cells. This herpesvirus (the fifth to be described) was named Epstein-Barr virus (EBV) and, in 1968, Werner and Henle demonstrated that seroconversion to EBV occurred during the course of infectious mononucleosis (IM). Subsequent to the finding that EBV is the causative agent of IM, several additional clinical syndromes and malignant disorders have been linked to EBV infection.
EPIDEMIOLOGY
Since the 1970s, EBV has been recognized to transmit primarily through contact with oropharyngeal secretions. EBV also has been recovered from the genital tract, suggesting a sexual mode of transmission. EBV infection may be transmitted through blood transfusions in which latently infected B lymphocytes serve as the source of infecting virus.
Seroepidemiologic studies have demonstrated a wide variation in the age at which EBV infection is established. In many areas of the developing world, EBV infection occurs in early infancy, and by 2 years of age, more than 80% of children have experienced primary EBV infection. The majority of these infections are asymptomatic. Passively acquired maternal antibody apparently is protective, with the majority of primary infections occurring after 4 to 6 months of age. In some areas of the developed world, primary infection does not occur until adolescence. In the United States, approximately 10% to 15% of susceptible college students become infected each year, and the majority of these infections are associated with a mononucleosis syndrome.
PATHOGENESIS
Epstein-Barr virus (EBV), a member of the Herpesviridae family, is an enveloped virus with 172 kb linear double-stranded DNA genome that codes for approximately 100 proteins. EBV infects over 90% of the world’s population and is spread by contact with oropharyngeal secretions from a virus carrier. The tonsils appear to be the initial site of viral entry and replication, but the virus persists throughout life in infected B lymphocytes. Whereas primary EBV infections usually are asymptomatic or only mildly symptomatic in young children, primary EBV infection in older children or adults may result in an infectious mononucleosis syndrome. EBV genome also has been detected in a variety of neoplasms, including Hodgkin disease, Burkitt lymphoma, and nasopharyngeal carcinoma, although EBV’s role in their pathogenesis is not well understood. EBV-associated lymphoproliferative disease also is a common cause of morbidity in individuals undergoing transplantation.
Persistent EBV infection likely results from a dynamic interplay between viral evasion strategies and host immune responses. Potent T-cell activation occurs and high levels of EBV-specific CD4+ and CD8+ responses are generated during acute EBV infection. How and why EBV persists despite these broad and vigorous immune responses is unclear, but recent studies have provided insight into potential EBV immune evasion strategies. An EBV IL-10 homologue (BCRF1) has been identified that has potent immunosuppressive activity. In addition, it appears that EBV exploits normal pathways of B cell differentiation to allow it to persist in a transcriptionally quiescent state in memory B cells and thus minimize immune recognition. EBV infection of resting naïve B cells occurs in the tonsillar mantle zone (Fig. 195.1) and results in generation of activated, proliferating B cell blasts through the expression of the viral growth program under the regulation of the transcription factor, EBV nuclear antigen 2 (EBNA 2). These EBV infected B cell blasts express many EBV proteins and are thus potentially subject to immune effector mechanisms, including lysis by EBV-specific CD8+ T-cells. However, some EBV-infected B cell blasts appear to escape immune surveillance and traffic to lymphoid follicles where they form germinal centers, turn off EBNA 2, and express a more restricted set of viral proteins (EBNA 1; latent membrane protein-1 or LMP-1; and latent membrane protein-2 or LMP-2). Survival of germinal center B cells normally requires signals from antigen on antigen presenting cells through the B cell receptor and on signals from CD40L on T helper cells through CD40. The expression of the EBV LMP-1
protein, a CD40 functional homologue and EBV LMP-2 protein, a B cell receptor functional homologue, may mimic these signals, allowing survival of the EBV-infected B cells and the acquisition of a memory B cell phenotype in which only limited EBV protein expression occurs. EBV-infected B cells leave the tonsil and circulate throughout the body as memory B cells with little transcriptional activity, thus likely escaping immune surveillance.
protein, a CD40 functional homologue and EBV LMP-2 protein, a B cell receptor functional homologue, may mimic these signals, allowing survival of the EBV-infected B cells and the acquisition of a memory B cell phenotype in which only limited EBV protein expression occurs. EBV-infected B cells leave the tonsil and circulate throughout the body as memory B cells with little transcriptional activity, thus likely escaping immune surveillance.
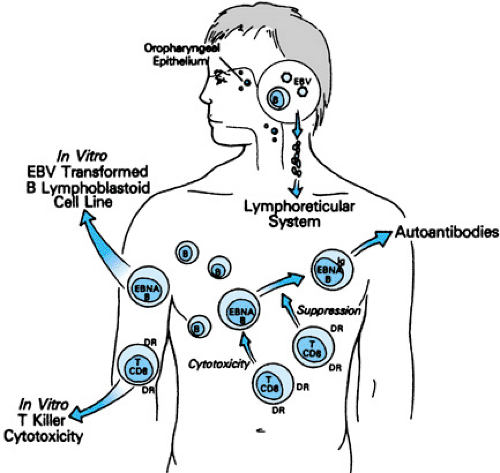 FIGURE 195.1. Immunopathogenesis of acute Epstein-Barr virus–induced infectious mononucleosis. DR, HLA-DR antigen; EBNA, Epstein-Barr nuclear antigens. |
During acute Epstein-Barr virus (EBV) infection in humans, up to 44% of peripheral blood CD8+ T cells stain with EBV viral epitope-specific tetramers; virtually all tetramer-staining cells express CD45RO, HLA DR, and CD38 suggesting high-level activation and turnover of these cells in vivo (Fig. 195.1). Interestingly, responses to EBV lytic proteins appear to predominate during acute infection. In healthy, chronically EBV-infected individuals, up to 5% of peripheral blood CD8+ T-cells stain with tetramers; responses to lytic and latent proteins have been detected. During acute infection, the majority of tetramer-staining cells co-stain with markers of cellular activation (HLA-DR, CD38) and turnover (Ki67); the co-expression of these markers decreases during convalescence. Although the majority of tetramer-staining CD8+ T-cells during acute infection are of the RO phenotype, tetramer-staining cells that co-express CD45RA (naive T-cell) or CD45RO (memory T-cell) are identified in convalescent individuals.
Activated natural killer (NK) cells also are observed in the peripheral blood of acute IM patients as also seen with other viral infections. NK cells are considered to play a primary role in the immune surveillance against virally or neoplastically modified cells, because they require no in vitro stimulation or previous antigen exposure. Interestingly, NK cells alone are not sufficient to counteract the establishment of EBV-transformed B cell lines in vitro; however, they do contribute to improved B lymphoblastoid cell line regression in the presence of EBV-specific cytotoxic lymphocytes (CTL). This effect by NK cells may be due to either direct cytotoxicity or IFN production, because EBV and EBV-infected cells induce gamma-IFN production by NK cells, and alpha-IFN is known to inhibit EBV-induced B-cell proliferation in vitro.
Biron et al. described a patient with complete absence of natural killer cells who experienced an unremarkable EBV infection, but experienced several life-threatening infections with VZV, CMV, and HSV, thus suggesting that NK cells are not necessary for control of primary EBV infection.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
Primary Epstein-Barr Virus Infections in Infants and Children
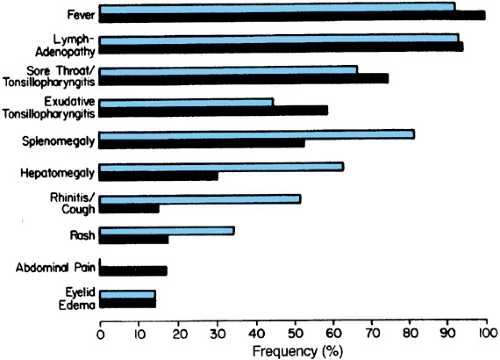
Primary EBV infections in young infants and children are frequently asymptomatic. When symptoms do occur, a variety of syndromes have been observed, including otitis media, diarrhea, abdominal complaints, upper respiratory infection, and IM (Fig. 195.2). These syndromes may occur without the production of heterophil antibodies, and EBV-specific serologic studies may be necessary to establish the diagnosis. Several studies have focused on the clinical and serologic findings that are associated with IM in infants and children. In those children with more than 50% mononuclear cells and more than 10% atypical lymphocytes, primary EBV infection was documented in the majority. Most of the children had clinical evidence compatible with IM (significant cervical adenopathy and tonsillar pharyngitis). Respiratory symptoms were frequently prominent, especially in young infants. Complications of EBV IM occur in approximately 20% of children, usually during or shortly after the peak of clinical illness. Table 195.1 lists the frequency of significant complications noted in one series of patients. Only 25% of infants 10 to 24 months of age demonstrated heterophil antibody responses, whereas 75% of children aged 24 to 28 months tested positive for heterophil antibody and only 60% of infants demonstrated VCA-IgM antibodies, compared with 100% of older children and young adults.
The IM syndrome is not uncommon among young children. The presence of atypical lymphocytes and lymphadenopathy in
the absence of heterophil antibodies further documents the age-related differences in host responses to EBV. We have demonstrated that young infants can mount an EBV-specific cytotoxic T-lymphocyte response during acute EBV infection. These cytotoxic T-lymphocyte responses in young infants appear to be directed against the same epitopes recognized by young adults.
the absence of heterophil antibodies further documents the age-related differences in host responses to EBV. We have demonstrated that young infants can mount an EBV-specific cytotoxic T-lymphocyte response during acute EBV infection. These cytotoxic T-lymphocyte responses in young infants appear to be directed against the same epitopes recognized by young adults.
TABLE 195.1. COMPLICATIONS PRESENT IN CHILDHOOD EPSTEIN-BARR VIRUS INFECTIOUS MONONUCLEOSIS | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Infectious Mononucleosis in the Adolescent
The IM syndrome appearing in adolescents is characterized by fever, anterior and posterior cervical lymphadenopathy, exudative pharyngitis, and fatigue. The syndrome is self-limited, lasting an average of 2 to 3 weeks. An estimated 30% to 50% of students entering college in the United States are susceptible (seronegative) to EBV. Approximately 10% to 15% of seronegative persons become infected each year, and the majority of those infected show signs and symptoms of classic IM. Studies of West Point cadets have demonstrated an association of clinically apparent IM with the likelihood of being under stress.
Individuals experiencing acute IM may develop morbilliform rashes when treated with ampicillin or penicillin during the acute phase of the disease. Hepatosplenomegaly is commonly present and severe, but only rarely results in splenic rupture, after trauma, and fulminant hepatitis secondary to periportal necrosis. One of the most common causes of hospitalization during acute IM is severe pharyngitis with concern for airway obstruction. This complication, which usually resolves in 24 to 72 hours, may be severe enough to warrant empiric treatment with intravenous corticosteroids, although the efficacy of such treatment is not proved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree