Entrapment Neuropathies of the Upper Extremity
Jenny T. Bencardino
Zehava Sadka Rosenberg
Entrapment neuropathies are characterized by alteration of nerve function secondary to compression by mechanical or dynamic forces. Anatomically narrow passages predispose individual nerves to these neuropathies. The compression can be acute, chronic, or intermittent. Not infrequently compressive neuropathies are related to space-occupying lesions such as tumors, cysts, inflammatory processes (rheumatoid arthritis, tuberculosis), or posttraumatic conditions (hematoma, myositis ossificans, and fracture callous formation). In some instances, changes of the surrounding soft tissues, such as thickening of connective tissue or postoperative scarring, can produce signs and symptoms of entrapment neuropathy. Edema, caused by hormonal changes associated with pregnancy, use of oral contraceptives, menopause, and hypothyroidism, is another known cause. Dynamic changes within a narrow space or “tunnel” during repetitive daily activity can create compression of a nerve with only minimal anatomic variations.1
Clinical manifestations of entrapment neuropathy include nonspecific signs and symptoms, such as:
Muscle weakness, with or without associated sensory loss
Sharp burning pain and paresthesia along the distribution of the affected nerve (Fig. 12.1)
Muscle atrophy and vegetative disturbances in advanced cases
Electromyographic and nerve conduction studies may show abnormal response to nerve stimulation. However, the deep course of some nerves (e.g., the posterior interosseous nerve in the proximal forearm) may produce unreliable electromyographic analysis. In most instances, conservative measures, including immobilization, local heat, and anti-inflammatory medications, are sufficient for the treatment of compressive neuropathies. More severe cases may require percutaneous steroid injections or surgical release of the nerve.
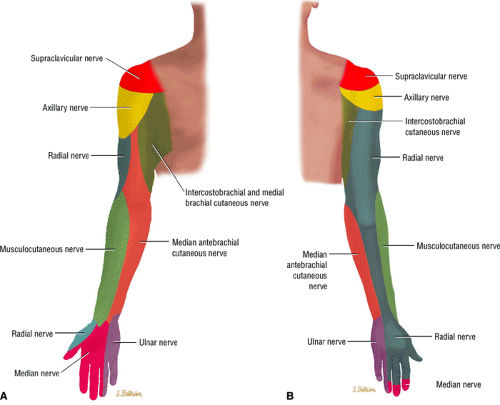 FIGURE 12.1 ● (A) Upper extremity volar sensory innervation. (B) Upper extremity dorsal sensory innervation. |
With the exception of MR imaging, most imaging techniques are insensitive for detecting compressive neuropathies. Conventional radiographs and computed tomography (CT) may show bony changes such as exostosis, osteophytes, fracture callus, and anatomic osseous variants that may produce nerve entrapment. Direct visualization of the nerve, however, is best achieved with MR imaging. Advantages of MR imaging in the diagnostic workup of neuropathy include:
Confirmation of the presence of nerve compression or entrapment
Determination of the presence of mechanical compression (e.g., soft-tissue mass)
Determination of the nature, site, and extent of the nerve compression
Exclusion of other lesions that can cause similar signs and symptoms (e.g., rotator cuff tear)
Normal peripheral nerves are seen on MR images as structures of low to intermediate signal intensity surrounded by fat, in specific anatomic locations. Mildly increased signal is a normal finding on water-sensitive sequences. Changes in signal intensity, size, and position of the involved peripheral nerve are valuable MR findings supportive of the finding of compressive neuropathy. Osseous and soft-tissue lesions can also be well depicted with MR imaging. In the subacute stage of muscle denervation, MR imaging typically shows interstitial T2 hyperintensity in the affected muscle group. In chronic cases, produced by a long-standing compressive neuropathy, muscle atrophy with fatty infiltration takes place.2,3, Increased muscle signal on fluid-sensitive images superimposed on muscle atrophy indicates an ongoing, partly reversible process.
Pathophysiology of Nerve Disorders
Pearls and Pitfalls
Terminology
Neuropraxia is a first-degree nerve injury with temporary loss of nerve conduction.
Axonotmesis is a second-degree nerve injury characterized by interruption of the axon with intact connective tissue structures surrounding the axon and Schwann cells.
Neurotmesis is complete disruption of the nerve and supporting connective tissue structures.
Nerve disorders have been classified according to the severity of injury and its potential for reversibility:
Neuropraxia, or first-degree nerve injury, is the least severe nerve injury with the greatest capability for recovery. There is a temporary loss of nerve conduction secondary to distortion of the myelin near the nodes of Ranvier due to ischemia, mechanical compression, or electrolyte imbalance. Also, there is a segmental block of conduction without Wallerian degeneration. Complete recovery is expected within 2 to 3 months.
Axonotmesis, or second-degree nerve injury, implies a more extensive injury due to interruption of the axon with secondary Wallerian degeneration distal to the site of injury. There is, however, preservation of all the supporting connective tissue structures that surround the axon and Schwann cells. The recovery period, ranging from weeks to months, depends on the distance between the site of injury and the end organs. The prognosis is relatively good, dependent on sprouting and re-innervation.
Neurotmesis refers to the most severe degree of injury, with no chance of regeneration. It is caused by complete disruption of the nerve and its supporting structures.
Sunderland4 further divided neurotmesis into three categories:
Third-degree nerve injury, in which the endoneurium is disrupted with intact perineurium and epineurium
Fourth-degree nerve injury, in which all neural elements are disrupted except the epineurium
Fifth-degree nerve injury, in which the nerve is transected, resulting in complete discontinuity of the nerve
Neurotmesis is an indication for surgical treatment with end-to-end anastomosis or nerve grafting. The surgical outcome depends primarily on the chronicity of the process.
Several categories of nerve injury may coexist in the same nerve. The double-crush theory maintains that a compressive lesion at one point along a peripheral nerve causes internal derangement of nerve cell metabolism and therefore lowers the threshold for occurrence of compression at another site.5
MR Imaging Techniques
Pearls and Pitfalls
Imaging Techniques
STIR or FS PD FSE images are more sensitive than T2 FSE images in the detection of denervated muscle.
Clinical assessment of peripheral neuropathy routinely involves nerve conduction studies and electromyography. As mentioned, however, MR imaging provides additional information to clinical neurophysiologic investigations and is, in addition, operator-independent and painless. MR examination has the further advantage of providing a lasting detailed topographical map of neuropathic involvement, avoiding localization errors of muscles in electromyography. Using electromyography as the gold standard, inversion recovery MR sequences have been shown to have a relative sensitivity of 84% and specificity of 100%.6
It remains to be determined whether nerve conduction or MR studies are the optimal diagnostic technique for entrapment neuropathies. At present, however, nerve conduction tests are more routinely used than MR. MR imaging studies are most often used to:
Exclude focal mass lesions or external compression
Identify signs of neuritis/neuropathy
Visualize denervation muscle injury
Acute axonal nerve lesions are manifested by T2 hyperintensity and increased girth of the nerve at and distal to the site of injury correlating with Wallerian degeneration and nerve edema. Proximal increased girth may also be encountered. Prolongation of the T2 relaxation time of denervated muscle fibers and post-gadolinium enhancement most likely reflect changes in the intramuscular vascular bed leading to capillary engorgement and increased muscular blood volume.7,8
Inversion recovery sequences have proven more sensitive than T2-weighted fast spin-echo imaging in the detection of T2 prolongation in denervated muscle. These changes can be
seen as early as 24 hours after complete transection of the sciatic nerve in rats and precede muscle atrophy.9 MR imaging has also been successfully used experimentally as an indicator of nerve healing and re-innervation following transection and repair of the posterior tibial nerve in rats. Changes in T2 relaxation, noted in the first 4 weeks following surgical intervention, completely returned to normal at 6 weeks of follow-up.10
seen as early as 24 hours after complete transection of the sciatic nerve in rats and precede muscle atrophy.9 MR imaging has also been successfully used experimentally as an indicator of nerve healing and re-innervation following transection and repair of the posterior tibial nerve in rats. Changes in T2 relaxation, noted in the first 4 weeks following surgical intervention, completely returned to normal at 6 weeks of follow-up.10
The use of intravenous paramagnetic contrast agents might also prove to be valuable in assessing entrapment neuropathies. Sugimoto et al.11 used dynamic MR imaging to study nerve damage and suggested symptoms of carpal tunnel syndrome are produced by circulatory disturbances in the median nerve caused by chronic hypoxia rather than by compression alone. They proposed that dynamic MR imaging could be used to distinguish normal from abnormal nerves by their different patterns of enhancement. Novel contrast media, such as superparamagnetic iron oxide, may potentially be useful in detecting macrophage invasion into the degenerating nerve distal to an axonal lesion.12
The use of Gadofluoride M-enhanced MR imaging also shows promise in the assessment of nerve regrowth and regeneration. This agent selectively accumulates and persists in nerve fibers undergoing Wallerian degeneration causing T1 shortening, which is present as early as 48 hours after nerve injury. Increased T1 signal disappeared on follow-up studies, correlating with the regrowth of nerve fibers. Interestingly, Gadofluoride M enhancement persisted in nonregenerating, permanently transected nerves.13
Entrapment Neuropathies of the Shoulder
Pearls and Pitfalls
Shoulder Neuropathies
Suprascapular nerve syndrome
Proximal entrapment associated with supraspinatus and infraspinatus muscle denervation
Distal entrapment associated with infraspinatus muscle denervation
T1- or PD-weighted images are required to assess fatty atrophy.
FS PD FSE images are sensitive to muscle denervation.
Axillary neuropathy includes quadrilateral space syndrome and posttraumatic injury to the axillary nerve.
The Parsonage-Turner syndrome is primarily associated with suprascapular nerve disease, although the axillary, radial, or phrenic nerves or the entire brachial plexus may be affected.
Associated Normal Anatomy
Suprascapular Nerve
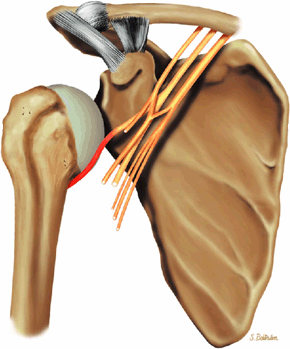
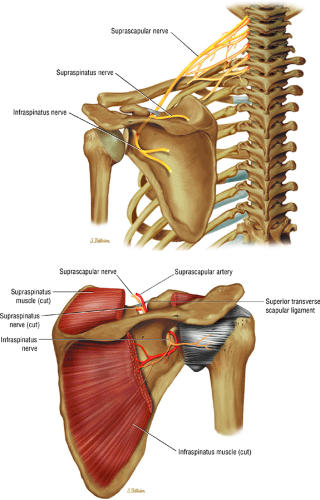
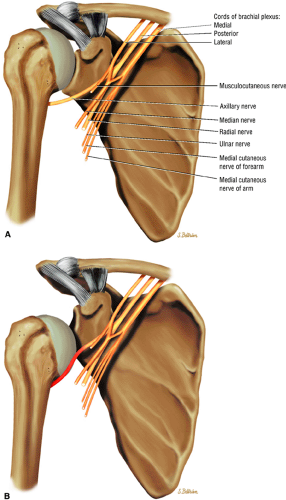
The suprascapular nerve originates from the upper trunk of the brachial plexus and receives fibers from the C5 and C6 nerve roots (Fig. 12.2). The suprascapular nerve contains motor fibers that innervate the supraspinatus and infraspinatus muscles as well as sensory fibers that carry sensation from both the glenohumeral and acromioclavicular joints. The nerve traverses the supraclavicular fossa with the suprascapular vein and artery. It then enters the suprascapular notch, making a sharp turn around the scapular spine. The transverse scapular ligament bridges the scapular notch or incisura superiorly, creating a fibro-osseous tunnel. The suprascapular vessels travel above the notch. At the scapular incisura, the supra-scapular nerve branches into the supraspinatus and infraspinatus nerves. In about 50% of the population, a second ligament, the spinoglenoid ligament, produces a second more inferior and posterior tunnel traversed by the infraspinatus nerve.1,14,15
MR Appearance
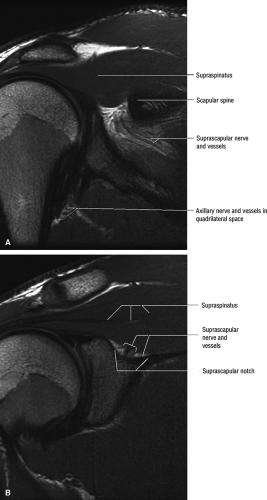
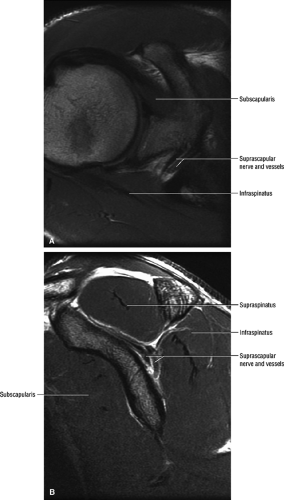
The suprascapular nerve is best identified on oblique coronal T1-weighted images within the supra-scapular notch, which is located at the junction of the glenoid with the scapular neck just medial to the superior glenoid rim. Fat within the notch outlines the nerve and accompanying vessels. The nerve then takes a sharp turn inferiorly, entering the spinoglenoid notch (Fig. 12.3). At this level, the supra-scapular neurovascular bundle is best visualized on axial MR images (Fig. 12.4). Prominent suprascapular veins are not infrequently noted accompanying the nerve and in some instances are responsible for the compressive neuropathy.
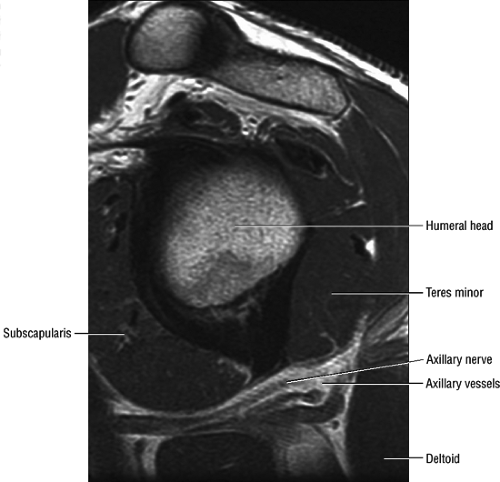
Axillary Nerve
The axillary nerve, the terminal branch of the posterior cord of the brachial plexus, receives contributions from C5 and C6 nerve roots. After its formation, the nerve courses dorsal to the axillary artery, along the anterior surface of the subscapularis muscle, where it takes a sharp turn posteriorly to travel along the inferior glenohumeral joint surface.16 The axillary nerve and posterior circumflex artery then enter the quadrilateral space, which is limited by the long head of the triceps brachii muscle medially, the teres minor muscle superiorly, the teres major muscle inferiorly, and the medial aspect of the proximal humerus laterally. The axillary nerve gives off four branches in the quadrilateral space: two motor branches to the anterior and posterior portions of the deltoid muscle, a sensory branch (the superior lateral brachial cutaneous nerve), and a motor branch to the teres minor muscle.17 Price et al. found that the branch to the teres minor and the branch supplying the lateral cutaneous innervation lie closest to
the glenoid rim.17 Using statistical analysis, these investigators showed that the axillary nerve travels at a fixed distance of approximately 2.5 mm from the inferior glenohumeral ligament throughout its course. An articular branch of the axillary nerve supplies the shoulder joint capsule.
the glenoid rim.17 Using statistical analysis, these investigators showed that the axillary nerve travels at a fixed distance of approximately 2.5 mm from the inferior glenohumeral ligament throughout its course. An articular branch of the axillary nerve supplies the shoulder joint capsule.
MR Appearance
The axillary nerve is best visualized on oblique sagittal T1-weighted images of the shoulder (Fig. 12.5). The axillary neurovascular bundle is seen below the inferior glenoid rim traversing the space between the teres minor muscle superiorly and the teres major inferiorly. Oblique coronal images oriented perpendicular to the proximal humeral shaft demonstrate the quadrilateral space adjacent to the medial humeral cortex and lateral to the long head of the triceps muscle.
Neuropathology of the Shoulder
Suprascapular Nerve Syndrome
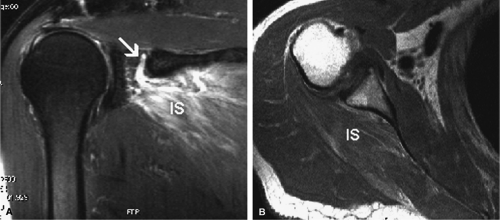
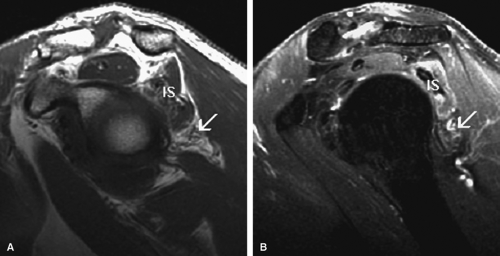
Given its peculiar branching pattern, proximal entrapment of the suprascapular nerve at the scapular incisura results in a supraspinatus and infraspinatus muscle denervation syndrome. Distal entrapment at the spinoglenoid notch may be manifested as isolated compromise of the infraspinatus muscle.18
Common causes of compression or entrapment of the supra-scapular nerve or its branches include:19,20,21
Suprascapular ligament stretching from repetitive scapular motion
Scapular fractures or other direct trauma
Posttraumatic calcification of the suprascapular ligament
Adduction and internal rotation caused stretching of the suprascapular nerve underneath the spinoglenoid ligament14
Overhead activities (e.g., painters, electricians, volleyball and tennis players), which may result in chronic mechanical stretching and irritation of the suprascapular nerve
Rotator cuff injury (Vad et al.,19 using electrodiagnostic testing, studied patients with full-thickness tears of the rotator cuff presenting with shoulder muscle atrophy. They found a relatively high prevalence (28%) of peripheral nerve injury, including suprascapular neuropathy, axillary neuropathy, and cervical radiculopathy.)
Iatrogenic injury to the suprascapular nerve during rotator cuff repair
Soft-tissue masses, osseous tumors, and vascular malformations, which can also compress the nerve along its course (Fig. 12.6)
Ganglion cysts at the scapular incisura, typically associated with superior and posterior labral tears (Fig. 12.7)
Antoniou et al. studied a series of 53 patients and found that pretreatment electromyographic findings were predictive of treatment response.22 Minimal electromyographic changes in patients with suprascapular nerve impingement were associated with limited response to treatment, especially in those with nerve compression secondary to spinoglenoid notch cysts.22 In the absence of an anatomic cause, management should be nonoperative; most patients will have a good to excellent result with physical therapy. Suprascapular notch cysts and spinoglenoid notch cysts respond significantly better to operative treatment.22,23 Traumatic lesions, including traction and direct closed injuries, have an equal response to operative and nonoperative treatment. Overall, the final outcome for traumatic injuries was significantly worse than for any other etiologic processes.22
MR Appearance
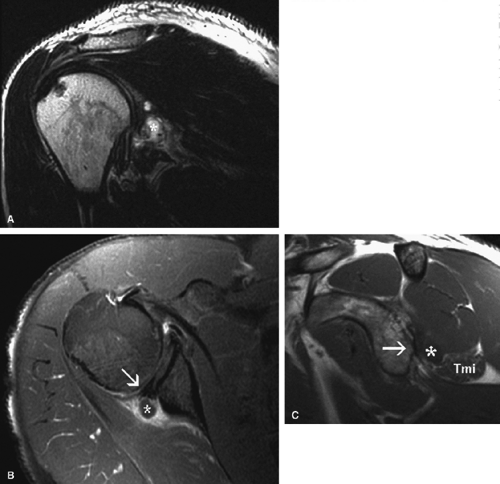
MR is a useful modality for the diagnosis of suprascapular nerve entrapment. On fat-suppressed T2-weighted MR images, involved muscle displays hyperintense signal (see Figs. 12.6 and 12.7). The site of entrapment can be inferred by the location of muscle involvement.24 Isolated denervation edema of the infraspinatus muscle places the site of entrapment at the spinoglenoid notch. When increased signal of both the supraspinatus and the infraspinatus is noted, compression at the suprascapular notch is most likely.
Using electromyography as the gold standard, Ludig et al. have shown that the sensitivity and specificity of MR in detecting suprascapular nerve entrapment vary depending on the MR criteria used:25
Using muscular denervation edema, sensitivity and specificity were shown to be 94.5% and 100%, respectively.
Using fatty changes and muscle atrophy (decreased muscle bulk, best seen on sagittal oblique spin-echo T1-weighted images), sensitivity and specificity were 81% and 80%, respectively.
Using fatty infiltration of the supraspinatus and/or infraspinatus muscles, sensitivity and specificity were 25% and 96%, respectively.
Axillary Neuropathy
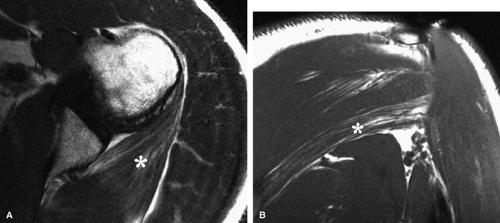
The quadrilateral space syndrome is caused by compression of the axillary nerve in the quadrilateral space. Fibrous bands are believed to be responsible for compression of the nerve and the posterior circumflex humeral artery as they travel within the quadrilateral space. Mass lesions, such as large paralabral cysts, can also damage or compress the axillary nerve within the quadrilateral space.26 Clinically, patients may have poorly localized shoulder pain, paresthesias, and discrete point tenderness in the lateral aspect of the quadrilateral space.27 In advanced cases, atrophy of the teres minor and the deltoid muscles can occur.
There may be several etiologies for post-traumatic axillary neuropathy:
In anterior shoulder dislocation, axillary nerve injury results from traction and compression of the nerve and the subscapularis muscle by the dislocated humeral head (Fig. 12.8). The shoulder is the most common joint dislocation seen in the emergency department and has an overall incidence of 1.7% in the population.28 Nerve injury complicates shoulder dislocation in up to 45% of cases,29 and the axillary nerve is the most commonly involved because of its relatively tethered course through the quadrilateral space.
The risk for axillary nerve and brachial plexus injury is greater if the shoulder is not reduced within 12 hours. Although neurologic injury is well known to orthopaedists who treat shoulder dislocation, few reports in the radiologic literature stress the association of teres minor atrophy with prior shoulder dislocation.30,31,32 Axillary nerve injury may develop after manipulative reduction in which traction with rotation or abduction is simultaneously applied.33
Posttraumatic injury to the axillary nerve can also be secondary to proximal humeral fractures34 and rarely as a result of a direct blow to the deltoid muscle.
The vast majority of patients recover with nonoperative treatment.35 Surgical débridement of fibrous bands has been reported to be successful in alleviating symptoms in patients with quadrilateral space syndrome who do not respond to conservative measures.36 In the management of posttraumatic axillary neuropathy, baseline electromyographic studies should be obtained within 4 weeks of the injury, with a follow-up evaluation at 12 weeks.35 If no clinical or electromyographic improvement is noted, surgery may be appropriate. The results of operative repair are best if surgery is performed within 3 to 6 months of the injury. The monofascicular composition of the nerve and the relatively short distance between the zone of injury and the motor endplate both contribute to the generally good outcome of axillary nerve repair.35
MR Appearance
Anatomically, the lateral cutaneous branch and the branch to the teres minor are closest to the glenoid rim and are therefore the most vulnerable.16 Clinical signs of axillary neuropathy are often vague. Wong and Williams found sensory deficits only in 182 of 196 patients with postoperative axillary neuropathy.37 Damage to the teres minor branch, however, may be difficult to assess on clinical grounds. MR evaluation of vague clinical symptoms may demonstrate signs suggestive of teres minor denervation injury (Figs. 12.9 and 12.10). Unlike electromyography, which directly evaluates nerve function, MR imaging provides indirect indicators of nerve injury by detection of changes in the fat and water composition of muscle. Changes in T1 and T2
prolongation can be appreciated within 15 days of the injury.36 The following MR findings are helpful in evaluating axillary neuropathy:
prolongation can be appreciated within 15 days of the injury.36 The following MR findings are helpful in evaluating axillary neuropathy:
Denervation muscle edema, not observed as often as fatty infiltration and atrophy
Teres minor fatty infiltration and decreased muscle bulk with or without involvement of portions of the deltoid.36 The incidence of atrophy or abnormal signal in the teres minor muscle has been reported to be as low as 0.8%.36
Associated abnormal MR findings such as labral tear, rotator cuff tear, subacromial subdeltoid bursitis, and acromioclavicular joint degenerative osteoarthritis
Parsonage-Turner Syndrome
Parsonage-Turner syndrome, also referred to as acute brachial neuritis, is clinically characterized by sudden onset of severe atraumatic pain in the shoulder girdle. The pain typically decreases spontaneously in 1 to 3 weeks and is followed by weakness of at least one of the muscles about the shoulder. The exact etiology has not been established, but viral and immunologic causes have been considered.38,39 The age range at presentation is quite wide, and there is typically a male predominance (male-to-female ratio of 2:1 to 11.5:1).40,41
In the early descriptions by Parsonage and Turner,40,41,42 the long thoracic nerve was reported to be the most frequently compromised nerve. However, later studies have shown a higher rate of isolated suprascapular nerve disease.39 The axillary, radial, and phrenic nerves,40 as well as the entire brachial plexus,41 may also be affected. Bilateral involvement is found in as many as one third of the patients.39 An abnormal electromyographic pattern with fibrillation potentials and positive waves may be seen.41
Parsonage-Turner syndrome can resemble a variety of other clinical entities, including rotator cuff pathology, cervical radiculopathy, spinal cord tumor, and peripheral nerve compression. The most confusing differential diagnosis is compressive neuropathy of the suprascapular or axillary nerve. The more insidious onset of pain and lack of spontaneous resolution of symptoms can help to distinguish compressive neuropathy from Parsonage-Turner syndrome. MR imaging can be useful in solving this diagnostic dilemma by displaying involvement of multiple muscles in more than one nerve distribution, characteristic of Parsonage-Turner syndrome.36 MR imaging (see below) also helps to exclude suprascapular or axillary nerve entrapment related to paralabral ganglions or other impinging mass lesions.43 Rotator cuff pathology can also be readily excluded using MR imaging.
No specific treatment has yet been proven effective in Parsonage-Turner syndrome. In the early stages, analgesics may be required. Steroids have proven ineffective for pain relief and do not improve muscle function. Rest is recommended, and immobilization of the affected upper extremity
may be helpful in pain relief and in preventing stretching of the affected muscles. Once the pain subsides, physical therapy is recommended. The overall prognosis is good, with almost 75% of patients experiencing complete recovery within 2 years. However, the period of time for complete recovery is variable, ranging from 6 months to 5 years.
may be helpful in pain relief and in preventing stretching of the affected muscles. Once the pain subsides, physical therapy is recommended. The overall prognosis is good, with almost 75% of patients experiencing complete recovery within 2 years. However, the period of time for complete recovery is variable, ranging from 6 months to 5 years.
MRI Appearance
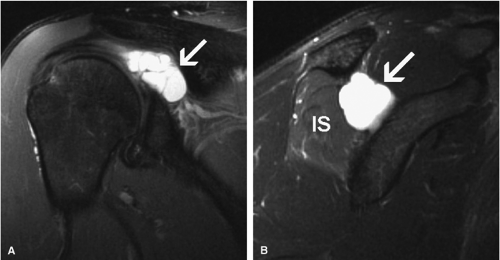
MR imaging findings in the acute stage of Parsonage-Turner syndrome include diffuse increased signal intensity on fluid-sensitive sequences consistent with interstitial muscle denervation edema.43 The most commonly affected muscles are those innervated by the suprascapular nerve, including the supraspinatus and infraspinatus (Fig. 12.11). The deltoid muscle can also be compromised in cases of axillary nerve involvement. Later in the course of the disease, muscle atrophy manifested by decreased muscle bulk may be visualized (see Fig. 12.11). Whole-body MR examination has been proposed as a potentially useful noninvasive diagnostic tool for Parsonage-Turner syndrome, allowing identification of a specific pattern of altered muscle signal confined to the shoulder girdle or a specific nerve distribution.44
Entrapment Neuropathies of the Arm and Elbow
Pearls and Pitfalls
Arm and Elbow Neuropathies
The cubital tunnel is the most common site of ulnar neuropathy.
Ulnar neuropathy also occurs at the arcade of Struthers, the edge of the medial intermuscular septum, the ligament of Struthers, the arcuate ligament, and the deep flexor pronator aponeurosis.
The pronator syndrome is the most common cause of median nerve entrapment. It is characterized by numbness in the median nerve distribution occurring with repetitive forearm pronation/supination.
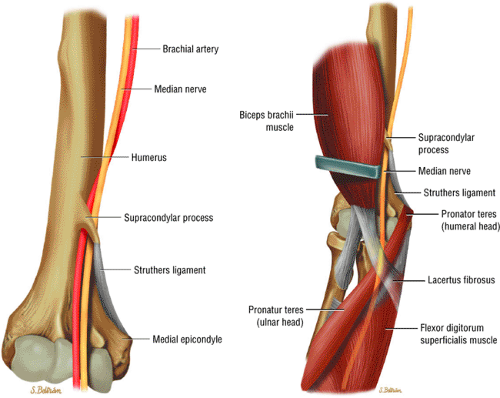
Potential sites of compression in the pronator syndrome include the supracondylar process/ligament of Struthers, the lacertus fibrosus, the pronator teres muscle, and the proximal arch of the flexor digitorum superficialis muscle.
The anterior interosseous syndrome affects only the motor branch of the median nerve and is less common than the pronator syndrome.
The radial tunnel syndrome involves compression of the posterior interosseous nerve without motor deficit.
The posterior interosseous nerve syndrome is a motor neuropathy with the same potential sites of compression as in the radial tunnel syndrome.
Associated Normal Anatomy
Ulnar Nerve
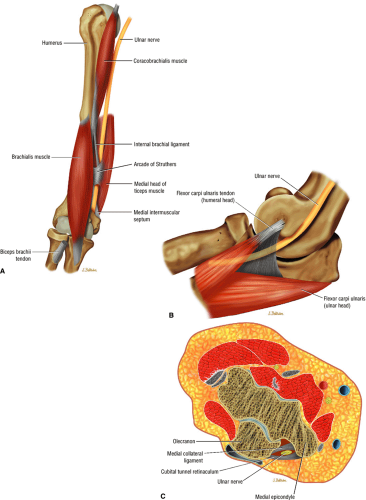
The ulnar nerve is the direct continuation of the medial cord of the brachial plexus. It contains motor and sensory fibers arising from the C8 and T1 nerve roots. At mid-arm level, the ulnar nerve crosses from the anterior compartment to the posterior compartment, piercing the intermuscular septum. Approximately 8 cm proximal to the medial epicondyle, the ulnar nerve may pass under the arcade of Struthers, which is present in 70% of individuals (Fig. 12.12).45 The arcade of Struthers is made up of fibers from the deep fascia of the distal arm connecting the medial intermuscular septum to the medial head of the triceps muscle.46
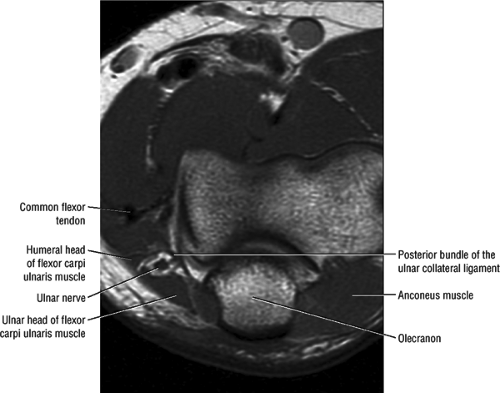
At the elbow, the ulnar nerve is located superficially as it descends posterior to the medial epicondyle within the cubital tunnel. The elbow capsule and portions of the ulnar collateral ligament form the floor of the cubital tunnel. Superficially, the roof of the tunnel is formed by the arcuate ligament (Osborne ligament or the cubital tunnel retinaculum), which extends from the medial epicondyle to the medial olecranon process (Fig. 12.13). O’Driscoll et al. postulated that the cubital tunnel retinaculum is likely a remnant of the anconeus epitrochlearis muscle.47 Normal anatomic variants range from no band at all to a well-defined muscle.
The ulnar nerve enters the anterior compartment of the forearm between the humeral and ulnar heads of the flexor carpi ulnaris. It then travels between the flexor carpi ulnaris
and the flexor digitorum profundus muscles in the forearm. It splits into superficial and deep branches at the wrist.
and the flexor digitorum profundus muscles in the forearm. It splits into superficial and deep branches at the wrist.
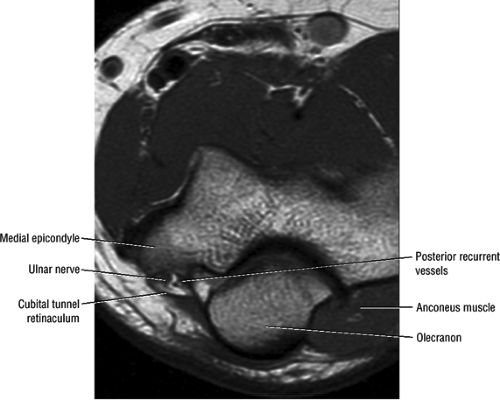 FIGURE 12.13 ● Normal MR anatomy of the ulnar nerve. Axial PD-weighted image demonstrates the ulnar nerve posterior to the medial epicondyle and medial to the posterior recurrent vessels. |
The ulnar nerve proper supplies the following structures:
The elbow joint
The flexor carpi ulnaris muscle and the ulnar half of the flexor digitorum profundus to the fourth and fifth fingers
The palmaris brevis (the superficial motor branch of the ulnar nerve)
The hypothenar muscles, the third and fourth lumbricals, all interossei, the adductor pollicis, the deep head of the flexor pollicis brevis, the flexor digiti minimi, the abductor digiti minimi, and the opponens digiti minimi (the deep motor branch of the ulnar nerve)
The medial palm
The palmar and distal dorsal skin of the fifth finger
The medial half of the fourth finger
MR Appearance
In most individuals, the ulnar nerve is clearly highlighted by fat throughout its course in the elbow and proximal forearm.48 Axial MR images depict the nerve traversing the cubital tunnel behind the medial epicondyle, roofed by the arcuate ligament (see Fig. 12.13). The accompanying vessels are usually located lateral to the nerve (see Fig. 12.13).49 In cases where the elbow has to be imaged in 90° of flexion, the size of the cubital tunnel is compromised, rendering identification of the ulnar nerve more difficult.49,50 Distal to the elbow joint, the ulnar nerve is commonly highlighted by fat as it travels between the ulnar and humeral heads of the flexor carpi ulnaris muscle and posterior to the flexor digitorum superficialis muscle (Fig. 12.14). The normal nerve is low in signal on T1-weighted images but typically demonstrates a mild increase in signal on fluid-sensitive sequences.
Median Nerve
The median nerve is formed by the blending of the lateral and medial cords of the brachial plexus, and it is the nerve to the radial side of the flexor portion of the forearm and hand (Fig. 12.15). It contains both motor and sensory fibers from the C5, C6, C7, C8, and T1 nerve roots. The median nerve descends in the arm, first lateral, then ventral, and finally medial to the brachial artery. It has no branches at the level of the arm. At the elbow, the median nerve is found medial and parallel to the brachial artery. It passes between the bicipital aponeurosis
(lacertus fibrosus) and the brachialis muscle, traveling deep to the pronator teres as it courses into the forearm (Fig. 12.16). In the elbow region, the median nerve supplies all the superficial muscles of the ventral forearm (the pronator teres, the flexor carpi radialis, the palmaris longus, and the flexor digitorum superficialis) except the flexor carpi ulnaris muscle.
(lacertus fibrosus) and the brachialis muscle, traveling deep to the pronator teres as it courses into the forearm (Fig. 12.16). In the elbow region, the median nerve supplies all the superficial muscles of the ventral forearm (the pronator teres, the flexor carpi radialis, the palmaris longus, and the flexor digitorum superficialis) except the flexor carpi ulnaris muscle.
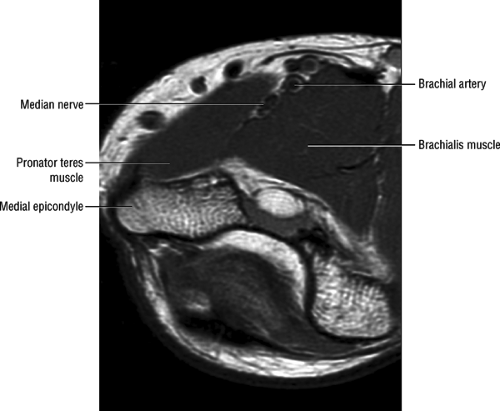 FIGURE 12.16 ● Normal MR anatomy of the median nerve. Axial PD-weighted image demonstrates the median nerve between the pronator and the brachialis muscles. |
The anterior interosseous nerve branches off the median nerve in close proximity to the bifurcation of the brachial artery into the radial and ulnar arteries, approximately 2 to 5 cm below the level of the medial epicondyle. The anterior interosseous nerve courses over the flexor digitorum profundus and along the interosseous membrane toward the wrist joint. It supplies all the ventral deep muscles of the forearm, including the radial half of the flexor digitorum profundus, the flexor pollicis longus, and the pronator quadratus muscles. The ulnar half of the flexor digitorum profundus is supplied by the ulnar nerve.
The most common nerve variation of the median and ulnar nerves is the Martin Gruber anastomosis, occurring in 22% to 39% of the population.51,52 The anastomosis presents in two patterns:
A motor branch from the median nerve in the proximal forearm to the ulnar nerve in the middle to distal third of the forearm
A motor branch from the anterior interosseous nerve to the ulnar nerve
In patients with a Martin Gruber anastomosis, the median nerve supplies all the hand intrinsic musculature, which may produce confusing findings on clinical examination.