Entrapment Neuropathies of the Lower Extremity
Zehava Sadka Rosenberg
Conrado F.A. Cavalcanti
Three major nerves supply the lower extremity: the sciatic nerve, the femoral nerve, and the obturator nerve (Fig. 6.1).1 The distance that these nerves have to travel (Table 6.1) predisposes them to multiple potential entrapment sites and compression neuropathies. Although lower extremity neuro-pathies are a major cause of pain and functional impairment, they are often overlooked or misinterpreted,2 primarily because of the complexity of the clinical picture and the difficulty in distinguishing neuropathies from non-neurologic, more common causes for discomfort in the lower extremity. Other neurologic causes for lower leg symptoms such as disk disease and lumbar plexopathy can mimic peripheral entrapment neuropathies as well as coexist with them, further confounding the clinical diagnosis.
Various imaging modalities can aid in the assessment of nerve entrapments of the lower extremity. Plain radiographs and computed tomography (CT) are useful in assessing osseous abnormalities such as spurs and fracture fragments.3 Ultrasound (US) is a very promising technique for assessing lower entrapment neuropathies because of its accessibility, facility in distinguishing nerves from adjacent vessels, dynamic capabilities, and ability to image long nerve segments.3,4,5,6,7,8,9,10 US, however, requires a high level of operator expertise, and the images are usually difficult for the non-radiologist to interpret.
At present, magnetic resonance (MR) is the imaging modality of choice for directly visualizing nerves, depicting entrapment sites, and mapping denervation patterns.3,11,12 Although the status of MR examination in detecting entrapment neuro-pathies remains to be established, it is evident that, despite some conflicting reports, it can play a significant and complementary role to electrophysiologic studies.13,14,15,16,17,18 West demonstrated increased short tau inversion recovery (STIR) signal compatible with muscle denervation within 4 days after nerve resection, prior to electromyographic (EMG) evidence of disease.16 Fleckenstein, on the other hand, noted that MR imaging was less reliable in
mapping and monitoring subacute and chronic denervation.13 One study correlating quantitative EMG and MR studies of the anterior tibial muscle depicted a high correlation between the two techniques in chronic denervation.15 In this same study MR imaging depicted denervation in additional muscles that were not, however, studied with EMG. In another large study of 90 patients with clinical evidence of peripheral neuropathy, MR examination demonstrated a sensitivity of 84% and a specificity of 100% compared to EMG studies.17 In a small subgroup of patients, however, abnormal EMG activity was noted in more muscles or in the absence of muscle involvement on STIR MR images.17 Comparison of MR studies with EMG in 40 patients with neurogenic foot drop revealed agreement in 92% of the patients.18 In a small percentage of these patients, MR imaging detected more widespread muscle disease than found on the EMG studies. In one patient a follow-up EMG study confirmed the MR finding.18
mapping and monitoring subacute and chronic denervation.13 One study correlating quantitative EMG and MR studies of the anterior tibial muscle depicted a high correlation between the two techniques in chronic denervation.15 In this same study MR imaging depicted denervation in additional muscles that were not, however, studied with EMG. In another large study of 90 patients with clinical evidence of peripheral neuropathy, MR examination demonstrated a sensitivity of 84% and a specificity of 100% compared to EMG studies.17 In a small subgroup of patients, however, abnormal EMG activity was noted in more muscles or in the absence of muscle involvement on STIR MR images.17 Comparison of MR studies with EMG in 40 patients with neurogenic foot drop revealed agreement in 92% of the patients.18 In a small percentage of these patients, MR imaging detected more widespread muscle disease than found on the EMG studies. In one patient a follow-up EMG study confirmed the MR finding.18
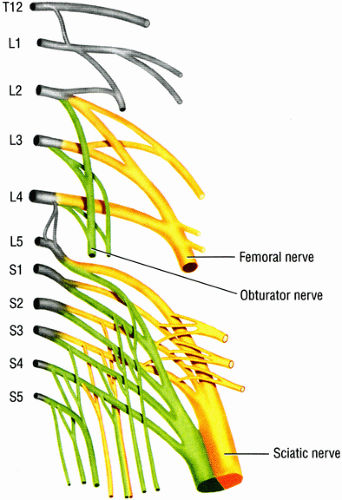 FIGURE 6.1 ● The origins of the three major nerves supplying the lower extremity: the sciatic nerve, the femoral nerve, and the obturator nerve. |
MR imaging may be particularly useful in diagnosing deep entrapment neuropathies, such as the piriformis muscle syndrome, in patients for whom EMG is problematic, such as patients taking anticoagulants, patients with bleeding disorders, patients who require serial studies, and children who have difficulty tolerating the pain associated with EMG.19,20,21 With the advent of recent technologic advances such as T2 neurography, it is likely that MR imaging will play an increasingly important role and may even, in certain settings, replace electrophysiologic studies for the detection of entrapment neuropathies of the lower extremity.
At present, there is relatively little information on the MR features of peripheral entrapment neuropathies of the lower extremity.3,12,22 MR imaging of neuropathies of the foot and ankle is particularly neglected. Even the normal anatomy of the nerves has been only briefly described.11,23 It is important to recognize the clinical and MR imaging features of entrapment neuropathies of the lower extremity, however, since early treatment, while the neurologic changes are still reversible, is crucial for full functional recovery. Knowledge of the detailed anatomy of the peripheral nerves and the muscles they innervate is indispensable.
Pathomechanism of Entrapment Neuropathies
Pearls and Pitfalls
Pathomechanisms
The etiology of neuropathy may be related to mechanical or dynamic causes.
Nerve entrapment may result in motor, sensory, and autonomic deficits.
Nerve injury is classified in order of severity from neurapraxia, to axonotmesis, to neurotmesis.
Most entrapment and compression neuropathies in the lower extremity are either mechanical, occurring at fibrous and fibro-osseous tunnels, or dynamic, related to nerve injury during specific limb positioning.24,25 Tumors, osteophytes, fracture fragments, scarring, aberrant muscles, and other mass-occupying lesions can cause local friction, a deviation in the course of the nerve, and subsequent entrapment. In many instances
the symptoms manifest only after a superimposed external force, such as an ill-fitting shoe, aggravates the nerve compression. Trauma, surgery, and ankle inversion injuries are known to cause stretch injuries to many of the nerves of the lower extremity. Hormonal changes, preexisting polyneuropathy, and systemic diseases such as diabetes mellitus and rheumatoid arthritis can also predispose to nerve entrapment.
the symptoms manifest only after a superimposed external force, such as an ill-fitting shoe, aggravates the nerve compression. Trauma, surgery, and ankle inversion injuries are known to cause stretch injuries to many of the nerves of the lower extremity. Hormonal changes, preexisting polyneuropathy, and systemic diseases such as diabetes mellitus and rheumatoid arthritis can also predispose to nerve entrapment.
Nerve entrapment can produce several types of signs and symptoms, including:
Motor function impairment, such as weakness, paralysis, and muscle atrophy
Sensory deficits, such as pain and paresthesias
Autonomic deficits, such as dry skin and ulceration
Treatment varies depending on the site of entrapment, the inciting cause, and the extent and chronicity of the process. Initially treatment is conservative, aimed at removal of the inciting agent by rest, behavior modification, footwear changes, orthotics, anti-inflammatory medications, and local steroid injection. If symptoms persist after 3 to 4 months of conservative management, surgical decompression is required to prevent permanent nerve damage and muscle atrophy. Complete nerve transection may require direct nerve repair or grafting.
Neuropathy is linked to endoneurial edema, increased endoneurial pressure, decreased blood flow to the nerve, and nerve ischemia.26 Demyelinization and neuropathy then follow. Nerve injury can be classified in increasing order of severity into neurapraxia, axonotmesis, and neurotmesis:27
Neurapraxia, or first-degree nerve injury, is an early and reversible segmental conduction block without axonal disruption ranging from transient ischemia (such as occurs from crossing the legs) to myelin sheath injury. Neurapraxia usually resolves within hours, days, or months with no residual sequelae.
Axonotmesis, or second-degree nerve injury, represents more advanced nerve damage, in which a focal disruption of the axon produces Wallerian degeneration distal to the site of injury. However, the intact investing connective tissues around the nerve allow axonal regeneration at a rate of approximately 1 to 2 mm a day, and the prognosis is still relatively good. Depending on the distance between the site of nerve injury and the muscle affected, axonotmesis can resolve within weeks to months.
Neurotmesis, or third-degree nerve injury, is characterized by an irreversible and complete axonal and connective tissue disruption. In addition to significant functional impairment, neurotmesis can produce painful neuromas secondary to unsuccessful attempts at nerve regeneration.
Axonotmesis and neurotmesis can be further subdivided into three categories depending on the number of disrupted surrounding connective tissue layers.28 More than one grade of injury frequently coexists within the same nerve. In general, early and mild nerve compression leads to neurapraxia, whereas chronic or more severe compression can lead to axonotmesis and neurotmesis.
MR Imaging Techniques
Pearls and Pitfalls
MR Imaging Techniques
Nerve visualization requires imaging in all three orthogonal planes using high-resolution T1-weighted images and fluid-sensitive fat-suppressed images (e.g., FS PD FSE or STIR).
MR intravenous contrast is used to assess mass lesions associated with nerve compression.
Normal nerves do not enhance with intravenous contrast administration.
MR T2 neurography selectively emphasizes fluid signal from nerves.
Chronic nerve damage is associated with fatty infiltration and decreased muscle bulk.
Partially reversible nerve damage displays increased signal on T1- and T2-weighted images.
In the double crush syndrome, a proximally compromised nerve is more susceptible to entrapment distally.
Distal entrapment affects the proximal nerve in the Valleix phenomenon.
Complete visualization of nerves requires imaging in all three planes. Nerves are optimally traced on axial MR images, and high-resolution axial T1-weighted images and fluid-sensitive images, ideally fat-suppressed, are recommended. Sagittal and coronal imaging should also be performed with fluid-sensitive sequences, preferably STIR.
Gadolinium contrast enhancement may be used to detect associated mass lesions that are compressing the nerve. Normal nerves do not enhance after intravenous contrast administration. Changes in the blood–nerve barrier and secondary leakage of gadolinium into the endoneurial space may account for the post-contrast enhancement of compressed nerves.26
A new gadolinium-based agent, Gadofluorine M (Gf), has shown promise in assessing acute nerve degeneration and regeneration in rats.29 Gf binds to degenerated nerves, resulting in nerve enhancement on T1-weighted images. Proximal-to-distal reversal of the enhancement has been noted in regenerating nerves. EMG studies are capable of assessing regeneration only once the nerve reaches the target muscle, and this process may take weeks to months. With Gf it is possible to assess nerve regeneration during the critical time interval in which a decision regarding nerve grafting must be made.
MR T2 neurography is a relatively new and promising technique for high-resolution imaging of peripheral nerves.19,20,21,30,31 The intermediate MR signal of a nerve is a mixture of signal from various nerve tissues, including endoneurial fluid, myelin, fatty interfascicular epineurium, and connective tissue. Neurography selectively highlights only the fluid signal from the imaged nerves using the following parameters:
A chemical shift-selective pulse (CHESS), which suppresses fat signal from around and within the nerves
High TE values (approximately 90 msec), which suppress signal from adjacent muscle
Radiofrequency saturation pulses, which suppress fluid signal from vessels
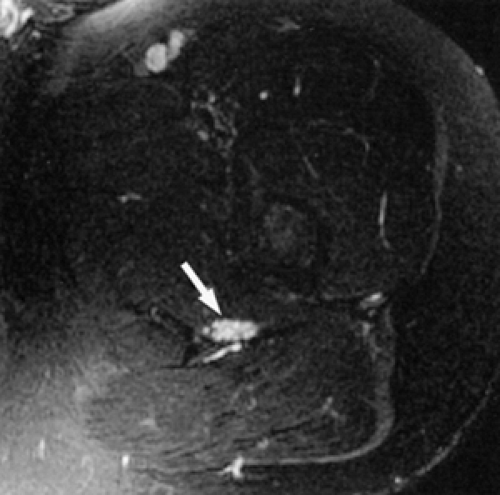
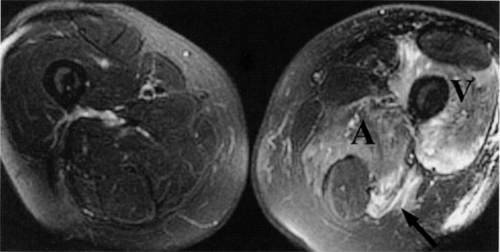
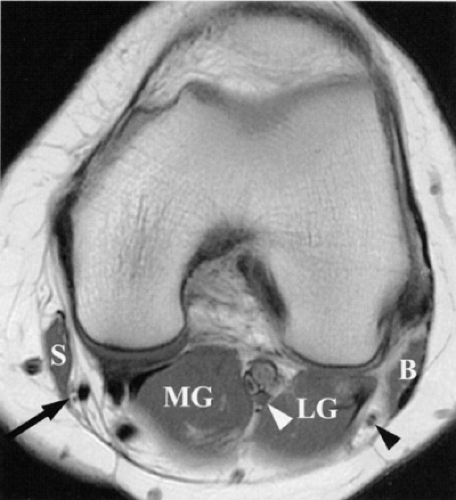
The only remaining signal is from endoneurial fluid, and the nerves stand out as bright signal intensity structures. MR neurography requires the use of phased-array surface coils and careful and constant magnet shimming. It provides excellent fascicular detail, best seen on axial images (Fig. 6.2). Further oblique image reformation and post-processing provide high-resolution selective images of the nerves along their longitudinal axis.
At present MR neurography is particularly useful for imaging large structures such as the lumbar and sacral plexi and the sciatic, femoral, and common peroneal nerves. The high-resolution images allow visualization of selective fascicular disease. Using this technique it is also possible to distinguish intraneural from perineural tumors. Smaller nerves, in the ankle and foot, however, are difficult to distinguish from adjacent bright signal vessels with neurography. Other disadvantages include the length of imaging time and sensitivity to motion artifact.21
Normal MR Anatomy of the Lower Extremity
Pearls and Pitfalls
Normal Anatomy
Peripheral nerves display intermediate signal intensity on T1- and PD-weighted images with a mild increase on fluid-sensitive sequences (signal intensity usually within the intermediate range on FS PD FSE images).
The fascicular anatomy of larger nerves demonstrates honeycomb morphology on axial images.
On T1-weighted images peripheral nerves demonstrate intermediate signal intensity, similar to muscle signal. Mildly increased signal intensity is noted on T2-weighted and other fluid-sensitive sequences, possibly due to the presence of endoneurial fluid within the fascicles of the nerve.3 The following MR characteristics are typical:
Nerves are seen as ovoid to round structures on axial MR images.
Longer segments of a nerve can occasionally be appreciated on coronal and sagittal images.
The fascicular anatomy of larger nerves demonstrates a honeycomb appearance, best appreciated on high-resolution axial MR images. This pattern is difficult to visualize in the smaller nerves.
On axial MR images, it is not uncommon to detect major divisions of the larger nerves before they actually branch away from each other. The peroneal and tibial divisions of the sciatic nerve can be visualized as they descend together in the thigh. Similarly, in the distal knee, the common peroneal nerve is subdivided into its two major branches, the superficial and deep peroneal nerves, long before the two divisions physically separate from one another.
Nerves usually travel within intervals between muscles and are typically well highlighted by fat. In young athletic individuals with bulky muscle mass, the nerves may be more difficult to detect because of the paucity of fat between muscle planes. Certain muscles can serve as landmarks for identifying adjacent nerves:
The saphenous nerve travels in close proximity to the sartorius muscle throughout most of its course in the thigh and knee.
The common peroneal nerve courses along the posteromedial aspect of the biceps femoris muscle.
Identifying the medial plantar nerve in the foot is made easy by following the flexor hallucis longus tendon.
Veins and arteries also aid in tracing the course of the nerves since they usually travel in the same fat plane. Conversely, nerves, particularly distally, are frequently difficult to distinguish from their adjacent vessels. On T1-weighted images nerves and vessels are isointense. On fluid-sensitive images both vessels and diseased nerves demonstrate increased signal. Familiarity with the spatial relationships between the nerves and the adjacent vessels, as well as the presence of flow void artifacts and tortuosity of the vessels, may aid in distinguishing between the two structures.
MR of Lower Extremity Nerve Pathology
The MR characteristics of peripheral nerve entrapment can be divided into two major categories:32,33,34
Direct evidence of nerve pathology consistent with alterations in the course, morphology, and signal characteristics of the affected nerve
Indirect signs of entrapment evidenced by muscle signal and size alterations compatible with denervation
Pearls and Pitfalls
Nerve Pathology
Nerve swelling and hyperintensity may represent an advanced process of nerve damage with fascicular and endoneurial edema.
Muscle denervation is associated with motor nerve entrapment.
On fluid-sensitive images hyperintense muscle signal represents subacute denervation (denervation edema).
Direct MR signs of entrapment include:
Course deviations. Mechanical deviation of the nerve from its normal course may be related to static conditions associated with mass effect such as bone spurs, scar, soft tissue tumors, ganglia, and aberrant muscles. These processes are easily depicted with MR imaging. Course deviation and entrapment of a nerve, however, can be dynamic and may be appreciated only in certain predisposing limb positions, such as foot pronation in medial plantar nerve entrapment or ankle plantar flexion and dorsiflexion in superficial peroneal nerve entrapment secondary to a muscle hernia.2 These types of entrapments can be missed on routine MR studies and may require additional studies following changes in limb positioning.
Morphologic and signal changes. Size and signal alterations also represent direct MR evidence of nerve pathology. Nerve swelling is compatible with edema at the fascicular level and may be a late sign of disease. In the smaller nerves, however, size differences can be difficult to assess. When present, edema may accentuate the honeycomb fascicular appearance of the nerve. Conversely, focal effacement of the normal fascicular pattern may also be encountered.19 The increased nerve signal is believed to reflect endoneurial edema and may also represent a fairly advanced process. Since most normal nerves have mildly increased signal on fluid-sensitive images, assessment of signal alterations can be challenging. The increased signal in asymptomatic individuals is usually mild and also involves the whole course of the nerve, whereas pathologic increased signal is typically focal and is usually found in areas susceptible to entrapment.
Indirect MR signs of entrapment include:
Muscle denervation. Motor nerve entrapment can be inferred when there is MR evidence of muscle denervation. Muscle denervation is characterized by signal alterations seen on both T1- and T2-weighted images. These alterations serve to localize the site of entrapment and provide information on the extent of motor damage.18 Bright signal on T2-weighted images is consistent with a fluid shift, due to muscle cell atrophy, from intracellular to extracellular compartments without a significant increase in the total water content of the muscle.35 Increased blood perfusion to the muscle may also contribute to denervation signal alterations. Increased muscle signal on fluid-sensitive images has been labeled as acute denervation edema, although strictly speaking it may be more accurate to refer to it as subacute denervation. Reversal of the muscle signal to normal may be encountered once the entrapment has resolved.16,36
Fatty infiltration. In chronic nerve damage there is fatty infiltration and decreased muscle bulk indicative of relatively irreversible muscle atrophy. Chronic denervation is depicted as bright muscle signal on T1-weighted images. Often, ongoing nerve damage manifests as increased signal on T1- and T2-weighted images. This constellation of findings indicates an ongoing partially reversible process. Rarely, muscle pseudohypertrophy related to excessive fatty infiltration and true muscle hypertrophy have also been noted with chronic denervation.37
In general, acute or subacute denervation is easily distinguished from other causes of increased T2 muscle signal such as contusions, musculotendinous injuries, infection, rhabdomyolysis, and compartment syndrome. Denervation edema is usually diffuse and interstitial and involves the whole muscle. It spares the fascial planes between muscles and adheres to a particular nerve distribution.
A few concepts should be kept in mind when assessing peripheral nerve entrapment on MR images: the double crush theory, the Valleix phenomenon, selective muscle involvement, variability in innervations, and the distance between the site of nerve injury and the affected muscle.
The double crush syndrome is a phenomenon in which a proximally compromised nerve has a decreased threshold for injury and as a result is more susceptible to entrapment distally.2,25,38,39,40,41 As a result, it is not uncommon to find two sites of entrapment present simultaneously. Spinal stenosis, for example, predisposes to nerve entrapment more distally. Simultaneous entrapment of the medial plantar nerves at both the tarsal tunnel and at the foot may also occur.
In the Valleix phenomenon, a distal entrapment affects the more proximal nerve.2,25,42 This process may, in fact, be the same as the double crush syndrome. Both entities can produce an odd distribution of muscle denervation signal, and the concomitant presence of two entrapment sites should be carefully looked for.
Selective muscle involvement is a common occurrence that manifests clinically, electrodiagnostically, and on MR examination as selective damage of only a few muscles along a specific nerve distribution. Sciatic nerve injury, for example, typically affects the peroneal division and spares the tibial division, partly because the former is more superficial, has larger fascicles and less connective tissue, and is fixed at two places. Similarly, entrapment of the common peroneal nerve usually affects the deep peroneal nerve more than the superficial peroneal nerve because the former is in closer proximity to the fibular head. This selective fascicular involvement can also occur within a single nerve branch,
and its etiology is not always known.19 On MR images it is depicted as signal alterations in only a select group of muscles along a single nerve distribution. For example, it is not unusual for only the plantaris and popliteal muscles to depict muscle denervation changes in tibial nerve entrapment. Similarly, denervation signal isolated to the anterior tibial muscle with relative sparing of the extensor digitorum longus muscle is often depicted in common peroneal neuropathy. Neurography may illustrate greater involvement of isolated nerve fascicles.
There are many variations in muscle innervation and many communicating nerve loops in the lower extremity, particularly in the foot region.43 For example, the deep peroneal nerve may, in rare instances, supply muscles such as the adductor hallucis and flexor hallucis brevis, muscles typically innervated by the lateral and medial plantar nerves. This variability can affect the distribution of signal alterations within denervated muscles and may produce puzzling MR patterns. Familiarity with variations in innervation aids in interpreting unexpected muscle denervation signal alterations.
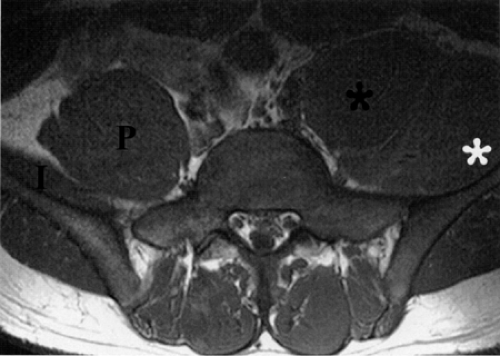
Finally, the distance from the site of entrapment to the innervated muscle should be considered when searching for muscle denervation abnormalities. Proximal damage to the peroneal division of the sciatic nerve may depict denervation signal in the leg or foot. This signal alteration may be missed if only the thigh is being imaged. Similarly, MR imaging of a painful foot may overlook a more proximal entrapment in the leg or thigh (Fig. 6.3). It is important to pay careful attention to signal changes on sagittal and coronal planes, where larger portions of the limb are illustrated, to help avoid this pitfall. If the clinical suspicion for entrapment is high and no abnormalities are noted on the initial study, imaging a more distal or proximal section of the limb can also be performed.
Sciatic Nerve
Pearls and Pitfalls
Sciatic Nerve
Sciatic neuropathy is associated with total hip replacements, especially revisions.
Sciatic neuropathy more commonly affects the peroneal nerve division.
The piriformis syndrome is related to spasticity, irritability, or inflammation of the piriformis muscle.
Normal Anatomy
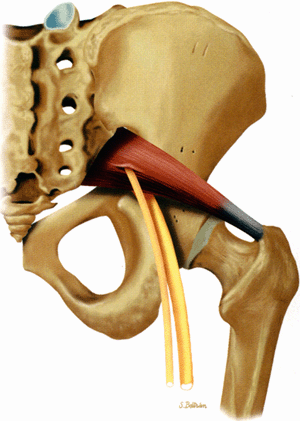
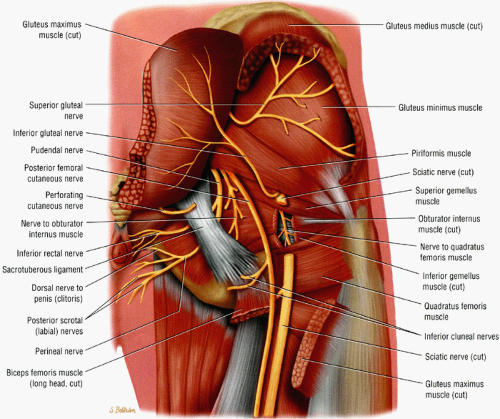
The sciatic nerve, the largest nerve in the body, receives contributions from the L4, L5, S1, and S2 nerve roots.1 When the nerve exits the greater sciatic foramen it is composed of two distinct tibial and peroneal divisions, enclosed by a common nerve sheath (Fig. 6.4). In the pelvis the sciatic nerve descends anterior to the piriformis muscle and courses downward into the thigh successively posterior to the superior gemellus, obturator internus, inferior gemellus, and quadratus femoris muscles (Fig. 6.5). The nerve continues down the thigh posterior to the adductor magnus muscle and the short head of the biceps femoris muscle and anterior to the gluteus maximus and the long head of the biceps femoris. In the distal third of the thigh the tibial and peroneal divisions physically separate into the tibial nerve and the common peroneal nerve.
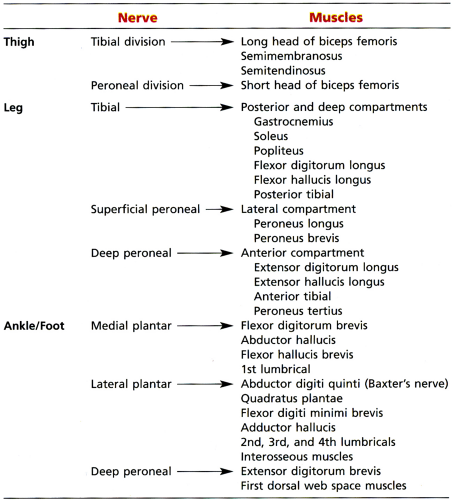
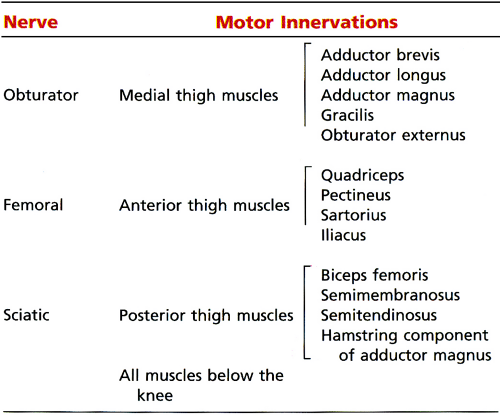
The sciatic nerve is one of the more important nerves in the lower extremity (Table 6.2). It provides knee flexion via innervation of the posterior thigh muscles and all sensory and motor functions below the knee (with the exception of sensation of the medial leg, which is supplied by the saphenous nerve, a branch of the femoral nerve) (Fig. 6.6). At the level of the hip, the tibial division of the sciatic nerve innervates the hamstring muscles, except for the short head of the biceps femoris muscle (which is innervated by the peroneal division). The tibial nerve provides all motor function to the posterior compartment of the leg and to the plantar muscles of the foot. The common peroneal nerve provides motor function to the anterior and lateral compartment of the leg and to the dorsum of the foot as well as sensation to the dorsum of the foot.
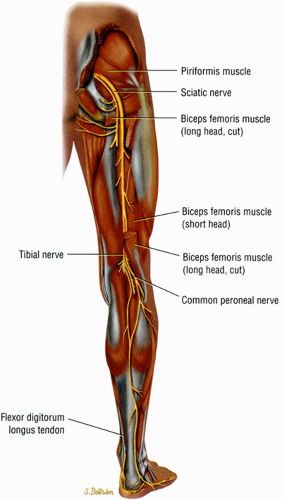 FIGURE 6.4 ● A posterior view of the right lower extremity demonstrating the sciatic nerve and its tibial and peroneal divisions. |
MR Appearance
Because it is large and surrounded by fat, the sciatic nerve is easily identified and can be followed as it descends through the pelvis and thigh. On axial MR images it appears as a large (up to 2 cm in diameter) ovoid structure of intermediate signal intensity. The fascicular pattern is clearly appreciated in all planes. The sciatic nerve often displays normally increased signal on all pulse sequences, which should not be mistaken for a fibrolipomatous hamartoma.
The sciatic nerve can be identified on axial, coronal, and sagittal plane images, but size, signal intensity, and relationship to adjacent soft tissue structures are best assessed on axial plane images. Coronal images are often useful in depicting the formation of the sciatic nerve from the L4–S2 nerve roots (Fig. 6.7). Oblique sagittal, axial, and coronal images using either the piriformis muscle21,44 or the sacrum as reference points have also been recommended for assessment of the sacral plexus and sciatic nerve. The oblique planes provide the advantage of visualizing a long segment of the nerve in a single image.
The following MR characteristics are typical:
The sciatic nerve is highlighted by fat as it courses in the greater sciatic foramen anterior to the piriformis muscle and lateral to the internal iliac vessels.
The nerve follows the course of the piriformis muscle and can be traced as it curves around the ischial spine into a more posterior and lateral location in the upper thigh. There the nerve is seen within an abundant stripe of fat between the greater trochanter and the ischial tuberosity (Fig. 6.8).
In the upper thigh the nerve is anterior to the gluteus maximus muscle and posterior, successively, to the superior gemellus, the obturator internus, the inferior gemellus, and the quadratus femoris muscles. Further down the nerve is anterior to the hamstring muscles and posterior to the adductor magnus muscle.
There is a gradual decrease in the amount of fat surrounding the nerve as it descends down the thigh. Finally, the nerve is flattened between the biceps femoris and the adductor magnus muscles.
In the lower thigh and proximal knee, as the adductor magnus muscle mass progressively disappears, the nerve is again highlighted by a significant amount of fat.
Not infrequently, the tibial and peroneal divisions of the nerve can be detected as they descend side by side in a common sheath before they diverge from each other in the proximal knee (see Fig. 6.8B).
The posterior femoral cutaneous nerve, located posterior to the sciatic nerve, can sometimes be identified as it migrates into a more superficial location in the upper thigh.
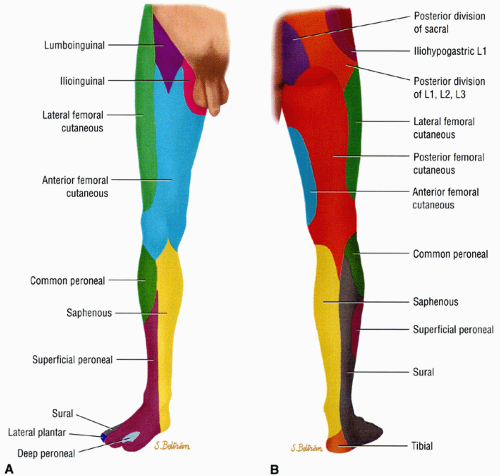 FIGURE 6.6 ● Anterior (A) and posterior (B) views of the sensory innervation of the lower extremity. |
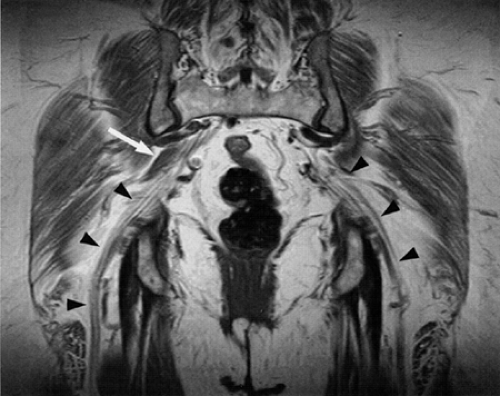 FIGURE 6.7 ● Coronal T1-weighted image of the sciatic nerve demonstrating the normal fascicular pattern of both sciatic nerves (arrowheads). A slip of the right piriformis muscle is noted (arrow). |
Sciatic Neuropathy
Injury to the sciatic nerve is a serious condition that can produce a flail leg and predispose to fractures and infections. Sciatic neuropathy can occur at the hip region or, less commonly, at the thigh. Sciatic neuropathy at the hip is most commonly iatrogenic and is associated with total hip replacements, usually after revision.45 The injury to the nerve is related to stretching or direct trauma and usually manifests clinically immediately after surgery. Delayed presentation is less common. The piriformis muscle syndrome (see discussion below) and the obturator internus muscle syndrome are additional causes of sciatic neuropathy.46,47,48,49,50,51,52,53 Sciatic neuropathy at the hip may also be secondary to hip fracture, vasculitis, arterial thrombosis, and cardiac surgery. Injury to the sciatic nerve usually spares the hamstring muscles since innervation to these muscles originates quite high.
Sciatic neuropathy more commonly affects the peroneal division of the nerve, with relative sparing of the tibial division. Factors contributing to the greater vulnerability of the peroneal division include:
The peroneal division is fixed at two points (at the sciatic foramen and at the fibular head), whereas the tibial division is fixed only at the sciatic foramen.
The peroneal division has larger fascicles with less protective surrounding connective tissue.
The peroneal division is more superficial and lateral than the tibial division.
Not surprisingly, sciatic neuropathy may be difficult to distinguish from the more distal common peroneal neuropathy. Symptoms of sciatic neuropathy vary depending on the level of injury and the relative involvement of the tibial and peroneal divisions. The most prominent feature is foot drop due to loss of innervation of the anterior tibial muscle (peroneal division). Loss of ankle and toe extension and foot eversion (peroneal division) and loss of ankle and toe flexion and foot inversion (tibial division) are other manifestations of sciatic neuropathy. Paresthesias, numbness, and a burning sensation in the lateral leg and dorsum of the foot (peroneal division)
and in the plantar surface of the foot (tibial division) are other manifestations of sciatic neuropathy.
and in the plantar surface of the foot (tibial division) are other manifestations of sciatic neuropathy.
Piriformis Muscle Syndrome
One of the best-known entrapment neuropathies of the sciatic nerve is the piriformis muscle syndrome, most often related to spasticity, irritability, or inflammation of the piriformis muscle. The existence of piriformis muscle syndrome, however, remains controversial, and the entity is probably overdiagnosed.53
Related anatomic features include:
The greater sciatic foramen is bordered superiorly and laterally by the sacrum and the iliac bone and by the sacroischial and sacrotuberous ligaments inferiorly and medially.
The piriformis muscle occupies a large part of the foramen as it extends from the sacrum to the greater trochanter (see Fig. 6.5).
A bursa is often found between the muscle and the bone.
The sciatic nerve traverses the greater sciatic foramen anterior to the piriformis muscle and is susceptible to compression due to loss of sciatic foramen volume, trauma, inflammation, local ischemia, and anatomic variations.
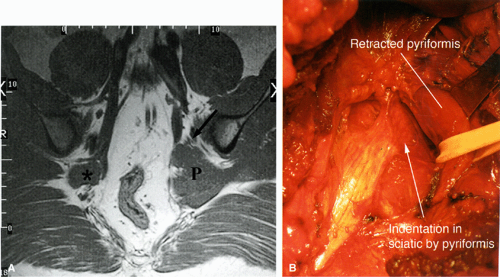
There are a number of causes of piriformis muscle syndrome. Piriformis muscle hypertrophy due to overstretching, hip flexion deformities, and lumbar lordosis may compromise the volume of the foramen and compress the sciatic nerve. Any disorders associated with increased spasticity, such as cerebral palsy, may also be associated with piriformis muscle syndrome.51 Piriformis muscle spasm secondary to sacroiliac disease, bursal distention, and inflammation or degeneration of the muscle/tendon unit are additional causes. Direct or indirect trauma to the sacroiliac or gluteal regions, hematoma, or fibrous adhesions can also decrease the volume of the foramen. Compromise of the vessels traversing the foramen and supplying the nerve can produce local nerve ischemia. Finally, anatomic variations such as a piriformis muscle pierced by the sciatic nerve or by the peroneal division of the sciatic nerve (Fig. 6.9), although present in a significant number of asymptomatic individuals, can also produce nerve compression, especially during contraction of the muscle. Piriformis muscle syndrome may also be caused by compression of the superior gluteal nerve.
The diagnosis of piriformis muscle syndrome should be entertained only after other causes of lower back and ischial pain, sciatic nerve compression elsewhere, and lower extremity pathology are excluded. Sacral or gluteal pain and tenderness over the belly of the piriformis muscle, aggravated by sitting, squatting, or walking and relieved by lying supine, is the most constant finding. In some patients the pain is aggravated when lying down at night or when getting up from a sitting position. Although infrequently seen, neurologic deficits may include a decrease in the ipsilateral ankle reflex, weakness in the knee and hip flexors, and numbness in the sciatic nerve distribution.51 Buttock pain and gluteal weakness and atrophy are associated with compression of the superior gluteal nerve.
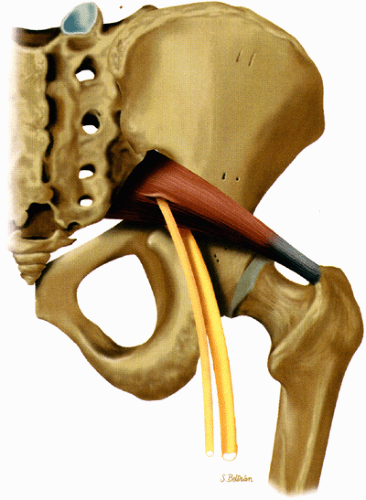 FIGURE 6.9 ● The piriformis muscle pierced by the peroneal division of the sciatic nerve (anatomic variation). |
The treatment of piriformis muscle syndrome is initially conservative, with anti-inflammatory medication, physical therapy, and corticosteroid and anesthetic injection into the muscle or bursal area. Surgical release of the superior gluteal and sciatic nerves and sectioning of the piriformis muscle may be attempted in refractory cases.
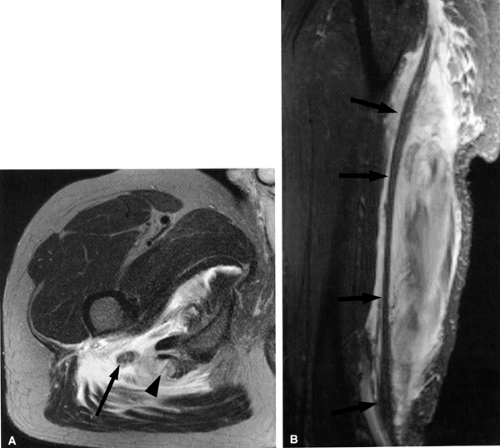
MR Appearance
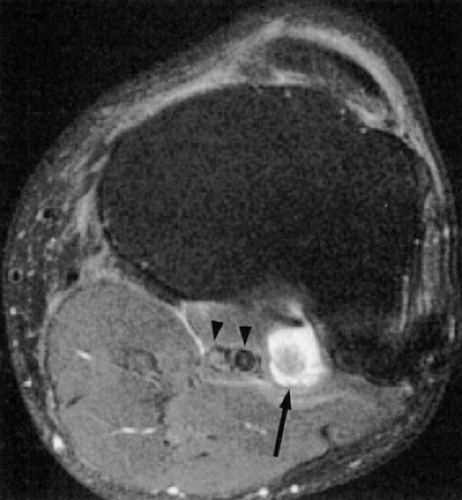
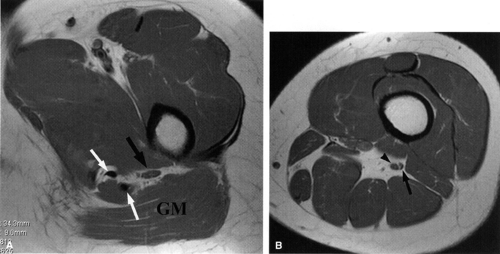
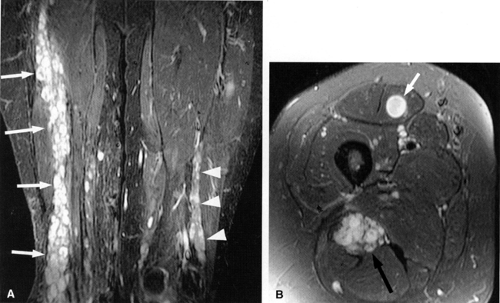
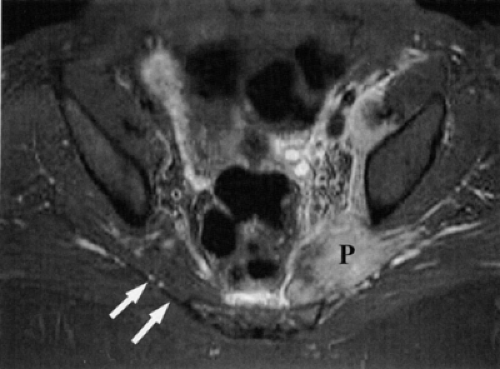
Direct MR evidence of sciatic neuropathy includes course deviation and increased size and increased signal of the nerve (see Fig. 6.2; Fig. 6.10). Obliteration of the fat planes around the nerve due to tumor, scar, or edema may also be noted (Fig. 6.11). In the thigh, muscle signal alterations consistent with denervation may be seen involving the hamstring muscles as well as the hamstring component of the adductor magnus muscle (Fig. 6.12). More distal signs of denervation may be missed on routine MR studies of the pelvis and hip since most muscles supplied by the sciatic nerve are located in the knee, leg, and foot. The distribution of affected muscles helps to distinguish sciatic neuropathy from more proximal plexopathy (Fig. 6.13). Varicosities, tumors, and inflammation displacing or engulfing the sciatic nerve can easily be detected on MR images (Fig. 6.14).44,48,54,55,56,57 Postsurgical complications
affecting the sciatic nerve such as amputation neuromas (Fig. 6.15), can also be evaluated on MR images.
affecting the sciatic nerve such as amputation neuromas (Fig. 6.15), can also be evaluated on MR images.
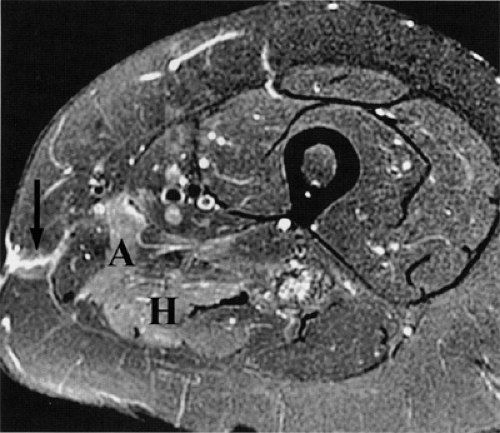 FIGURE 6.12 ● Denervation edema after resection of a sciatic nerve tumor. This axial T2-weighted image demonstrates denervation edema in the hamstring muscles (H) and adductor magnus muscle (A). |
The clinical diagnosis of piriformis muscle syndrome is often missed or delayed due to the rarity of the condition and the nonspecific symptoms.51 Initially, MR examination is used to assess other potential causes of low back, posterior hip, thigh, or buttock pain such as lumbar disk herniation, lumbar stenosis, and tumors. If MR examination of the lumbar spine is nondiagnostic and EMG for L5–S1 radiculopathy is normal, further MR examinations of the pelvis may be helpful in identifying pathologic changes. MR imaging has also been proposed as a follow-up tool to evaluate denervation in patients treated with botulinum toxin for piriformis muscle syndrome.49
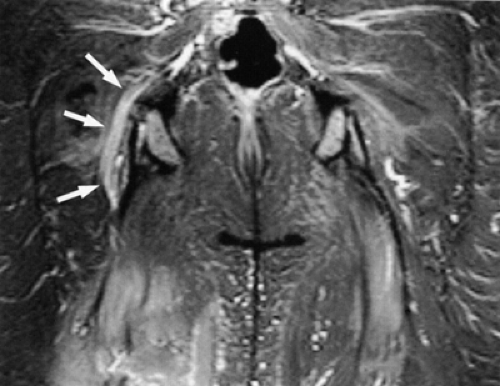
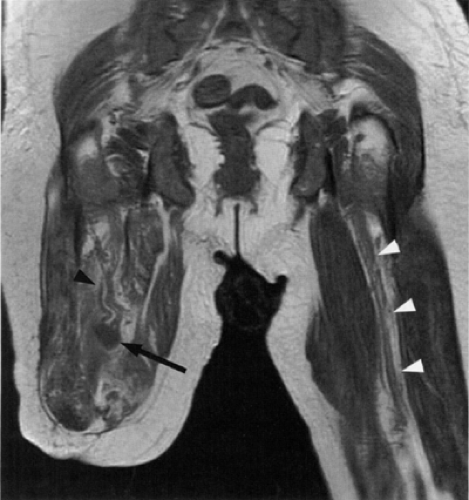
MR diagnosis of piriformis muscle syndrome is best made on pelvic images that display both piriformis muscles so that a comparison can be made. Axial and coronal images are both useful for assessing the size of the muscles. Characteristic MR findings include the following:
Significant asymmetry in the piriformis muscles is noted between the symptomatic and asymptomatic sides (Figs. 6.16 and 6.17). Mild asymmetry of the muscles, however, is often present in asymptomatic individuals.
Presence of selective atrophy49,50 or hypertrophy of the piriformis muscle and accessory muscle slips47,52
Detection of the sciatic nerve or one of its divisions within the substance of the piriformis muscle. An intramuscular location of the sciatic nerve, however, may be a normal variation in up to 10% of individuals.
Increased signal in the nerve and in the piriformis muscle may also be seen.49
MR neurography is a promising technique in the diagnosis of sciatic nerve pathology and piriformis muscle syndrome (see Fig. 6.2).19,21,58 In one study, MR neurography in 239 patients with non-discogenic sciatica demonstrated 93% specificity and 64% sensitivity for diagnosing piriformis muscle syndrome. Diagnosis was based on the concomitant findings of increased nerve signal at the sciatic foramen and asymmetry of the piriformis muscles.58 When muscle asymmetry was used as the only criterion, the specificity and sensitivity for piriformis muscle syndrome decreased to 66% and 46% respectively. Hypertrophy and less commonly atrophy of the piriformis muscle on the affected side were also noted.
Open MR-guided anesthetic injections of the piriformis muscle have been used to confirm the diagnosis of piriformis muscle syndrome and to guide treatment.58 Long-standing relief was noted in 23% of patients after one or two injections. Open MR-guided injections also proved more reliable than transvaginal blind or fluoroscopically guided injections of the piriformis muscle and have the added benefit of not exposing the patient to the ionizing radiation associated with CT-guided injections.58
Femoral Nerve
Normal Anatomy
The femoral nerve originates from the L2, L3, and L4 nerve roots of the lumbar plexus. It supplies motor innervation to all the anterior thigh muscles, with the exception of the tensor fascia lata, and sensation to the anterior and distal medial thigh, anteromedial knee, and medial leg and foot (Fig. 6.18).
Pearls and Pitfalls
Femoral Nerve
The saphenous nerve is the largest branch of the femoral nerve.
Femoral nerve compression frequently occurs underneath the inguinal ligament.
Anterior compartment (thigh) muscle atrophy is associated with femoral nerve entrapment.
Saphenous nerve entrapment occurs in the adductor canal.
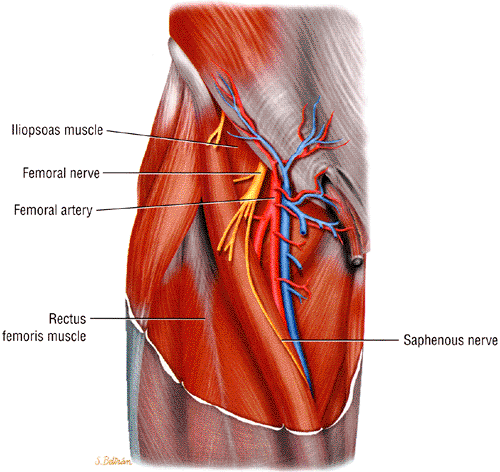
The femoral nerve initially travels under the psoas muscle and then courses anteriorly between the iliacus and psoas muscles, supplying them with motor innervation.1 It then enters the leg under the inguinal ligament. As the nerve passes under the inguinal ligament it travels in a relatively rigid tunnel, called the lacuna musculorum, the floor of which is formed by the iliac bone and iliopsoas muscle and the roof of which is formed by the iliopectineal arch (a fascia traversing from the iliopubic or iliopectineal eminence to the inguinal ligament) and the inguinal ligament. The nerve then enters the femoral triangle lateral to the femoral artery and divides into the superficial and deep branches. The superficial branch provides motor innervation to the pectineus and sartorius muscles and carries sensation from the anterior thigh. The deep branch provides motor innervation to the quadriceps muscles and sensation, via the saphenous nerve, to the medial thigh, leg, and foot. The saphenous nerve continues distally in an oblique fashion with the femoral artery and vein deep and parallel to the sartorius muscle (Fig. 6.19).
MR Appearance
The femoral nerve is best visualized on axial MR images of the pelvis and hips. It appears as an ovoid, intermediate-intensity structure on all pulse sequences. Compared to the sciatic and obturator nerves, the femoral nerve is more difficult to see on coronal or sagittal images of the pelvis. Depending on the amount of fat present, the nerve may be initially seen deep to the psoas muscle and then anterior to the iliopsoas muscle. The ease with which the nerve can be traced depends on how closely it adheres to the muscle. The nerve is usually easier to identify as it migrates anteriorly into a more superficial position in the lower pelvis, underneath the inguinal ligament. The latter structure may be seen as a fine, low-signal-intensity linear band that migrates medially on sequential axial images. As the femoral nerve approaches the upper thigh it traverses within the femoral triangle, a fat-filled superficial space also occupied by the more medially located femoral vein and artery. Often the nerve is depicted as a poorly defined structure composed of multiple intermediate-signal-intensity fascicles surrounded by fat (Fig. 6.20). The various terminal branches of the nerve can sometimes be seen as they split off from the main femoral nerve.
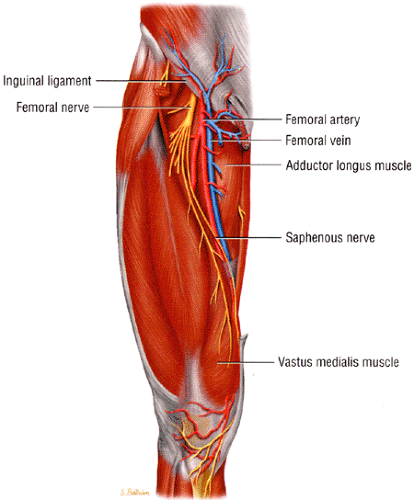
The largest branch of the femoral nerve, the saphenous nerve, can be followed in the thigh using the sartorius muscle and the femoral vein and artery as landmarks. The saphenous nerve is seen as a small, intermediate-signal-intensity, round structure within a fat plane, first posterior and then lateral to the sartorius muscle (Fig. 6.21). The nerve is accompanied by the femoral vein and artery and is bordered laterally and anteriorly by the vasti muscles. The nerve migrates, along with the sartorius, from a relatively anterior position in the proximal thigh to a posterior location in the knee area. The sartorial branch of the nerve can be followed as it pierces the fascia, between the sartorius and gracilis tendons, to become superficial at the level of the knee joint.59 At this location the sartorial branch is usually lateral (in 66% to 70% of cases) to the sartorius muscle but may also be found medial to the muscle. In the medial leg the branches of the saphenous nerve can be visualized within the subcutaneous fat along the great saphenous vein.
Femoral Neuropathy
Compression of the femoral nerve, known also as the iliacus muscle syndrome, commonly occurs underneath the inguinal ligament within the lacuna musculorum. Injury to the nerve is often iatrogenic,60,61,62,63 related to gynecologic procedures (e.g., hysterectomy), pelvic surgery, hip surgery/replacement, femoral artery catheterization, and arterial bypass procedures. The relatively superficial course of the nerve in the proximal thigh predisposes it to direct injury. Gunshot wounds, lacerations, and hip and pelvic fractures are additional causes of femoral nerve compression. Since the nerve traverses underneath the inguinal ligament together with the iliacus and psoas muscles, injuries to these muscles can produce femoral nerve injury. The nerve courses deep to the rigid iliac fascia, predisposing it to compressive and ischemic injury64,65,66 associated with iliacus muscle hematoma.
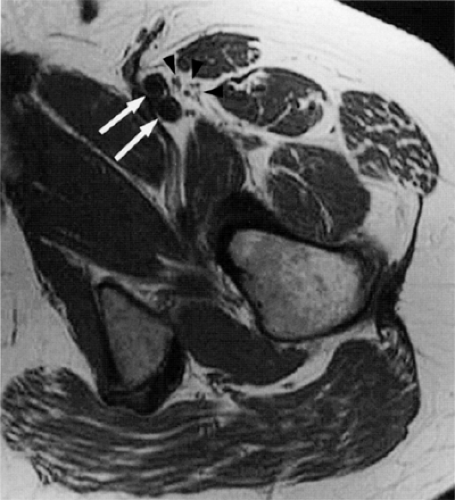 FIGURE 6.20 ● The normal femoral nerve in the upper thigh. Axial T1-weighted image demonstrating the normal bundles of the femoral nerve (arrowheads) lateral to the femoral vessels (arrows). |
Clinical indications of femoral neuropathy include:
Buckling and decreased knee extension (quadriceps weakness)
Decreased hip flexion
Difficulty arising from a sitting position and climbing up stairs (iliopsoas muscle weakness)
Atrophy of the muscles of the anterior compartment
Absent knee jerk
Sensory deficits in the anterior and medial lower thigh, and medial knee, leg, and foot
Entrapment of the saphenous nerve can occur in the adductor canal. The superficial location of the nerve in the leg predisposes it to lacerations and contusions. Saphenous nerve injury is the most common neurovascular injury after knee
arthroscopy and occurs in 7% to 22% of patients undergoing arthroscopic meniscal repair.59,67,68,69,70,71 Nerve injury has also been noted in patients with posterolateral instability, possibly related to stretching of the nerve.72
arthroscopy and occurs in 7% to 22% of patients undergoing arthroscopic meniscal repair.59,67,68,69,70,71 Nerve injury has also been noted in patients with posterolateral instability, possibly related to stretching of the nerve.72
Femoral neuropathy must be differentiated from lumbar plexus and L4 radiculopathy. Conservative treatment of femoral neuropathy should be attempted before surgical intervention, since the complications associated with surgery can have serious consequences.
MR Appearance
Most reports of entrapment neuropathies of the femoral nerve are related to traumatic injury of the iliopsoas compartment. CT and MR images show evidence of mass effect due to iliacus or iliopsoas muscle tears, hematomas (Fig. 6.22), and posttraumatic pseudoaneurysm of the iliac vessels.64,65,66 Femoral neuropathies secondary to iliopsoas compartment masses and distended iliopsoas bursa have also been described.73 The quadriceps muscles may display signal alterations related to denervation.
There are few descriptions of the imaging characteristics of saphenous nerve injury. Since the nerve is sensory, no muscle denervation changes are noted. In one report a neurilemmoma of the saphenous nerve was detected.74 Displacement of the saphenous nerve by a meniscal cyst has also been described on MR and US examinations.75 George et al. also reported finding a rare neuritis ossificans (heterotopic ossification) of the saphenous nerve, which presented on MR studies as a soft-tissue mass in the fat planes between the sartorius and vastus medialis muscles.76
Obturator Nerve
Normal Anatomy
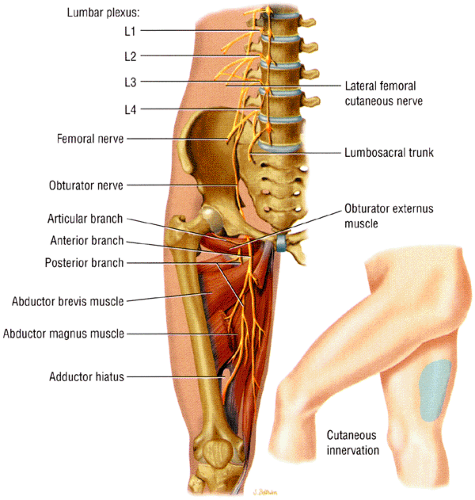
The obturator nerve supplies the medial thigh muscles. Spinal nerve contributions from L2, L3, and L4 roots fuse to form the obturator nerve within the substance of the psoas major muscle.1 The coalesced nerve emerges from the medial border of the psoas muscle and descends inferiorly and anteriorly along the iliopectineal line into the lesser pelvis (Fig. 6.23). The nerve exits the pelvis at the superior aspect of the obturator foramen via the obturator canal. Just prior to its exit from the pelvis, the nerve subdivides into its anterior and posterior divisions. Both branches traverse the obturator canal and pierce the obturator externus muscle. The anterior division descends posterior to the pectineus and adductor longus muscles and anterior to the obturator externus and adductor brevis muscles. The nerve supplies the hip joint and sends motor branches to the gracilis, adductor brevis, adductor longus, and occasionally pectineus muscles. It also supplies a patch of skin on the medial side of the thigh. The posterior branch descends posterior to the adductor brevis and anterior to the adductor magnus muscles. It supplies the knee joint, obturator externus muscle, adductor component of the adductor magnus muscle (the hamstring component is supplied by the sciatic nerve), and occasionally adductor brevis muscle. Variability in the obturator nerve and its branches77 and the presence of an accessory obturator nerve are common findings.
Pearls and Pitfalls
Obturator Nerve
Entrapment neuropathy is usually associated with trauma and postsurgical injury to the inguinal area and pelvis.
Pelvic tumors, including metastatic extension, may cause obturator neuropathy.
Groin pain in athletes is associated with involvement of the anterior branch of the obturator nerve distal to the obturator tunnel.
MR Appearance
The obturator nerve is best visualized on pelvic axial MR images, on which it appears as a round, low- to intermediate-signal-intensity
structure (Fig. 6.24). Occasionally, the nerve is seen on anterior coronal images (see Fig. 6.24B). The nerve can be traced as it originates from the lumbar spine and traverses inferiorly and anteriorly surrounded by a large amount of pelvic retroperitoneal fat. The nerve is located anterior to its accompanying vessels and can be followed as it dives under the superior pubic ramus to enter the obturator foramen. The anterior division of the nerve is depicted in the thin fat plane posterior to the pectineus and adductor longus muscles and anterior to the adductor brevis muscle. The posterior branch is identified in a fat stripe between the adductor brevis and adductor magnus muscles.
structure (Fig. 6.24). Occasionally, the nerve is seen on anterior coronal images (see Fig. 6.24B). The nerve can be traced as it originates from the lumbar spine and traverses inferiorly and anteriorly surrounded by a large amount of pelvic retroperitoneal fat. The nerve is located anterior to its accompanying vessels and can be followed as it dives under the superior pubic ramus to enter the obturator foramen. The anterior division of the nerve is depicted in the thin fat plane posterior to the pectineus and adductor longus muscles and anterior to the adductor brevis muscle. The posterior branch is identified in a fat stripe between the adductor brevis and adductor magnus muscles.