Overview of Epidemiology and Treatment Principles
Congenital differences of the hand and upper limb are common, affecting up to 1:626 live births.
1, 2, 3 and
4 The true incidence of abnormalities in upper limb differences is likely to be higher, owing to the association with other systemic anomalies resulting in fetal loss as well as the fact that many differences are so mild as to not command clinical recognition or evaluation. Familiarity with normal patterns of growth and developmental milestones is essential for the pediatric hand and upper extremity surgeon. Furthermore, understanding of the normal genetic mechanisms and embryology of the hand and upper limb is critical in understanding pathoanatomy, guiding surgical treatment strategies, and directing future efforts toward biologic solutions.
It is important to remember, in all of these discussions, that the ultimate goal of the pediatric upper limb surgeon is to maximize function and outcomes, while being sensitive to issues regarding aesthetic appearance, family dynamics, and social perception.
The purpose of this chapter is to provide a primer of hand and upper limb development—embryologic and genetic—and describe the language with which we classify congenital differences. While by no means an exhaustive scientific treatise, the information here will help to guide the surgeon in understanding principles for contemporary care as well as future investigation.
Limb Development and Embryology
You got to be careful if you don’t know where you’re going, because you might not get there.
—Yogi Berra
The hand and upper limb arise from a single limb bud, which comes from the lateral body wall at 26 days of gestation in humans (
Table 1.1).
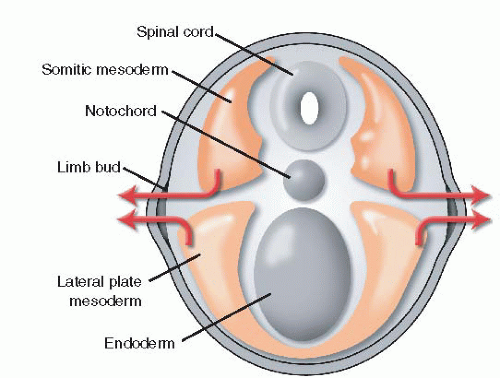
5 The limb bud, which is comprised of mesodermal cells covered in ectoderm, bulges at the junction of the dorsal and ventral surfaces. The mesoderm of the limb bud develops from both the somatopleure and the lateral plate. The somatopleural mesoderm contains the precursors of muscle, nerve, and blood vessels, while the lateral plate mesoderm forms bone, cartilage, and tendon (
Figure 1-2).
7Upper limb development then proceeds in a proximal-todistal fashion, ending at the 8th week of gestation, at which time all the structures of the upper limb have been formed. The mesenchymal cells coalesce to create a preskeletal blastema in the central portion of the limb bud. These cells then
differentiate into either chondrocyte or osteoblastic precursors. Chondrification begins with the humerus at 36 days of gestation and ends with the distal phalanges at day 50.
Joints develop by repression or regression of chondrogenesis (so-called cavitation), mediated by the WNT4, WNT14, and cartilage-derived morphogenetic protein-1 proteins. The shoulder joint appears at day 36 and proceeds until the formation of the interphalangeal joints at day 47.
5,8,9The muscles of the upper limb are formed and identifiable by the 7th week. In general, superficial muscles develop before deep muscles, and proximal muscles precede distal ones.
Nerve ingrowth begins at day 36, though the mechanisms by which this occurs remain unknown. The neural tube is the source of future sensory nerves, whereas the neural crest gives rise to motor branches.
The subclavian artery is apparent by the third week of gestation, and the arterial tree of the upper limb is present
at day 30. The median artery is the first to arise from the brachial artery. The ulnar artery develops shortly thereafter, forming the deep palmar arch in the hand. The radial artery is the last major distal branch to form, followed by the regression of the median artery.
10Hand formation begins with a single chondrogenic condensation at the end of the developing limb bud, surrounded by the precursors to tendon and muscle. With growth, this becomes the hand plate, and over time the rays form and are separated by flattened interdigital tissue. The hand plate appears at day 37, and the digital rays are identifiable by day 41. Through programmed cell death, or apoptosis, the interdigital tissue recedes, allowing formation of individual digits.
11 This process is initiated at day 47 and is completed by day 54 of gestation.
12 Digital separation proceeds in a distal-to-proximal fashion, explaining the phenomenon of incomplete simple syndactyly. The process of apoptosis is mediated by a number of gene products, including fibroblast growth factors (FGFs), Msx, and HOX7 (homeobox gene).
13, 14 and
15 Each digit has its own “identity,” according to its position on the hand plate. TBX2 and TBX3 are transcription factors that regulate bone morphogenetic proteins (BMPs), perhaps controlling digital formation and identity.
16,17
The Genetics and Molecular Biology of Limb Development
Be at the right place, at the right time, and do the right thing.
—Elton Hall
The process of normal hand and limb development is exquisitely complex and coordinated. In general, limb development can only proceed uninterrupted and undisturbed with three “rights”: the right proteins are regulated and secreted by the right cell(s) at the right time. Insights into the genetics and molecular biology of hand and upper limb development are, in essence, focused on understanding these three rights.
Three distinct signaling centers have been identified that guide and regulate limb development through cell-tocell interactions and release of growth factors and other molecules (
Table 1.2). Each signaling center is responsible for one axis of limb development: proximodistal, anteroposterior (radioulnar), and dorsoventral.
Much of the information about limb development, the role of varying signaling centers, and the factors they secrete comes from classic animal studies and human genetic evaluations, particularly those in which the factor or center of interest is removed. In general, theories about the role of certain genes or proteins are proven by three types of observations or experiments. First, a protein is deemed important for a component of development only if it is present in the correct cells at the correct time. This is typically done by in situ hybridization studies. Second, absence of a particular gene or gene product must result in failure of formation. This information is obtained via genetic manipulation of appropriate animal models (e.g., gene knockout mice) or careful genetic analysis of humans with congenital differences. Finally, normal development should be rescued or artificially induced by supplying the protein or gene product in question after it has been genetically or physically taken away. Again, technologic advances in genetic manipulation, selective gene expression, and embryonic handling have allowed such experiments to be performed.
The Apical Ectodermal Ridge and Fibroblast Growth Factors
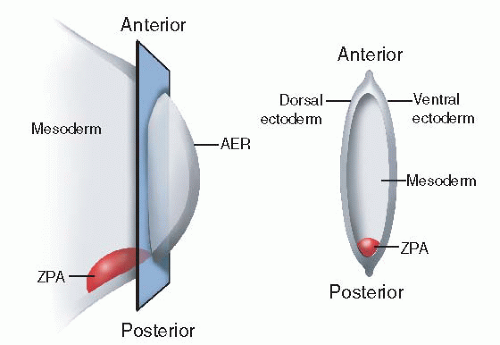
The apical ectodermal ridge (AER) or rim lies at the distal tip of the developing limb bud and guides proximodistal growth and development (
Figure 1-3). Just beneath the AER is the so-called progress zone. The progress zone contains cells of mesodermal origin. The current theory is that the longer cells remain in the progress zone, the more distal their ultimate differentiation location will be.
The AER is induced from epithelial-mesenchymal interaction, the exact mechanisms of which are under continued investigation. Part of this interaction involves the WNT signaling center, mediated by the fibroblast growth factor Fgf10.
18,19 Indeed, in the absence of WNT signaling, the AER and thus the limb fail to form.
20,21 This highlights the recurring concept that all three signaling centers continue to interact with one another to provide proportionate, appropriate growth.
Removal of the AER results in transverse failure of development and formation.
22,23 The earlier the AER is lost, the more proximal the transverse deficiency. Indeed, the inability to form an AER results in experimental amelia, supporting its role in proximodistal development.
20,21,24 Similarly, ectopic implantation of AER tissue results in formation of an extra limb at the implantation site.
5AER activity is mediated by several FGFs, including Fgf8, Fgf2, Fgf4, Fgf9, and Fgf17. Fgf8 is expressed by all cells of the AER and is therefore considered an AER marker. Addition of FGF can replace or duplicate AER function. For example, in experiments in which the AER is removed, provision of Fgf2, Fgf4, and Fgf8 can restore normal proximodistal growth.
23 Furthermore, additional sources of FGFs provided in the flank (interlimb) region may result in an extra, supernumerary limb.
25 Conversely, removal of FGF will simulate AER absence. Fgf10-deficient mice, for example, demonstrate complete transverse limb defects.
26Although FGF is the principal mediator of AER action, the underlying mesoderm beneath the AER provides signals to stimulate FGF production and secretion.
27 Again, timely interactions between signaling centers and adjacent cells are required for appropriate, proportional limb development.
Zone of Polarizing Activity and Sonic Hedgehog
The zone of polarizing activity (ZPA) is a cluster of mesodermal cells located on the
posterior border of the developing limb bud (
Figure 1-2). The ZPA guides anteroposterior (radioulnar) development.
28 The anteroposterior axis is the first to be defined, even before the limb bud is present. The initial description and insights into ZPA function were elucidated after classic grafting experiments, in which transplantation of posterior cells to the anterior aspect of the limb bud resulted in mirror-image ulnar duplications.
29 Furthermore, the number of abnormal digits was directly related to the number of ZPA cells transplanted.
30 Similar experimental results were obtained when retinoic acid was applied, which induced an additional or second ZPA to form.
31