Electrodiagnostic studies play an important role in the evaluation of patients suspected of having a myopathic disorder. They are used to exclude alternative diagnoses, confirm the presence of muscle disease, narrow down the differential, and identify an appropriate biopsy site. The most informative part of the electrodiagnostic study is needle electromyography. This allows for the analysis of spontaneous activity and motor unit action potential morphology and recruitment patterns. This article proposes a practical electrodiagnostic approach and describes the electrophysiologic patterns of the most commonly encountered myopathies.
Key points
- •
Electrodiagnostic studies are an extension of the physical examination.
- •
In the appropriate clinical setting, they are an important tool in the evaluation of patients with suspected myopathies.
- •
Electrodiagnostic patterns may help recognize the underlying pathophysiologic process and help direct further testing.
Introduction
The evaluation of patients suspected of having a myopathy begins with a thorough history and clinical examination. This process leads to the elaboration of a clinical impression, based on symptoms, progression, family history, and examination findings. Further diagnostic tests are then ordered using a hypothesis-driven approach to add laboratory evidence in support of or against the clinical suspicion. Electrodiagnostic (EDX) studies, in this respect, are an extension of the physical examination and may help establish the diagnosis of myopathy.
EDX studies, however, are not always needed to diagnose a myopathy. This is particularly true in the pediatric and, occasionally, the adult population. Oftentimes, patients with inherited myopathies present with characteristic phenotypes, and, possibly, a positive family history. In these cases, it is reasonable to proceed directly to genetic testing. In addition, at times, the diagnosis ultimately requires a muscle biopsy, regardless of the EDX study results. Therefore, if clinical suspicion for a myopathy is high, generally corroborated by elevated creatine kinase (CK) levels, it is often reasonable to skip or limit the extent of the EDX studies. Finally, EDX studies may be normal in selected muscle diseases (certain endocrine, metabolic, congenital, and mitochondrial myopathies). Thus, in the appropriate clinical context, normal EDX studies do not necessarily rule out the presence of a myopathy.
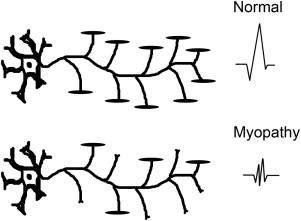
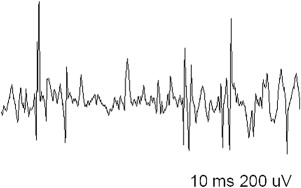
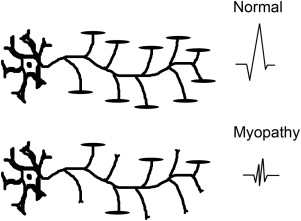
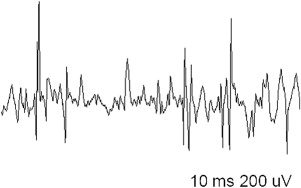
EDX studies are most useful to diagnose a myopathy when further data are needed to exclude alternative diagnoses, confirm the presence of a muscle disease, and narrow down the differential. The role of EDX studies is summarized in Box 1 . First of all, the results of nerve conduction studies (NCSs) and electromyography (EMG) are used to exclude neuromuscular conditions that may mimic a myopathy (such as motor neuron disease and neuromuscular junction disorders or, occasionally, motor neuropathies). Second, EMG is often able to confirm the diagnosis of a muscle disorder, when motor units with characteristic morphology and recruitment pattern are identified ( Figs. 1 and 2 ). In such cases, EMG may also add diagnostic information relating to the location, type, and severity of the underlying process. For example, the presence of abnormal spontaneous activity may help narrow down the differential among different myopathic processes ( Box 2 ). Finally, EMG may be useful in identifying target muscles for biopsy. This is particularly helpful when the only clinically weak muscles are not easily accessible for biopsy (such as the gluteal muscles, the hip flexors, or the paraspinals). The yield of a muscle biopsy increases when a weak (Medical Research Council grade 4 of 5), but not end-stage, muscle is biopsied. EMG analysis allows the evaluation of multiple sites and the identification of affected muscles that are not weak on neurologic examination.
- 1.
Exclude neuromuscular conditions that may mimic a myopathy
- a.
Motor neuron disease
- b.
Motor neuropathies
- c.
Neuromuscular junction disorders
- a.
- 2.
Provide EMG evidence of the presence of a myopathy (although EMG may be normal in the presence of selected myopathic processes)
- 3.
Characterize the myopathy
- a.
Location (proximal, distal, symmetric, or asymmetric)
- b.
Presence/absence of abnormal spontaneous activity
- c.
Severity
- a.
- 4.
Identify target muscles for biopsy
- 1.
Inflammatory myopathies (often)
- a.
Polymyositis
- b.
Dermatomyositis
- c.
Inclusion body myositis (IBM)
- d.
Immune-mediated necrotizing myopathy (with or without association with cholesterol-lowering agents)
- a.
- 2.
Toxic/necrotic myopathies (often)
- a.
Cholesterol-lowering agent myopathies (eg, statin myopathies)
- b.
Critical illness myopathy
- c.
Chloroquine/hydroxychloroquine
- d.
Amiodarone
- e.
Colchicine
- a.
- 3.
Muscular dystrophies, including the distal muscular dystrophies/myopathies, hereditary inclusion body myopathies, and the myofibrillar myopathies
- 4.
Congenital myopathies (some)
- a.
Nemaline rod myopathy
- b.
Centronuclear/myotubular myopathy
- c.
Central core myopathy
- d.
Multicore/minicore myopathy
- a.
- 5.
Myopathies associated with selected infectious agents
- a.
HIV and human T-lymphotropic virus 1–associated myositis
- b.
Trichinosis
- c.
Toxoplasmosis
- a.
- 6.
Metabolic myopathies (some)
- a.
GDS II (acid alpha-glucosidase deficiency or Pompe disease)
- b.
GSD III (debrancher enzyme deficiency)
- c.
GSD IV (branching enzyme deficiency)
- d.
Lipid storage myopathies
- a.
- 7.
Myotonic disorders
- a.
Myotonic dystrophy type 1 (DM1) and myotonic dystrophy type 2 (DM2)
- b.
Myotonia congenita, paramyotonia congenita (PMC), potassium-aggravated myotonias
- c.
Hyperkalemic periodic paralysis
- a.
Introduction
The evaluation of patients suspected of having a myopathy begins with a thorough history and clinical examination. This process leads to the elaboration of a clinical impression, based on symptoms, progression, family history, and examination findings. Further diagnostic tests are then ordered using a hypothesis-driven approach to add laboratory evidence in support of or against the clinical suspicion. Electrodiagnostic (EDX) studies, in this respect, are an extension of the physical examination and may help establish the diagnosis of myopathy.
EDX studies, however, are not always needed to diagnose a myopathy. This is particularly true in the pediatric and, occasionally, the adult population. Oftentimes, patients with inherited myopathies present with characteristic phenotypes, and, possibly, a positive family history. In these cases, it is reasonable to proceed directly to genetic testing. In addition, at times, the diagnosis ultimately requires a muscle biopsy, regardless of the EDX study results. Therefore, if clinical suspicion for a myopathy is high, generally corroborated by elevated creatine kinase (CK) levels, it is often reasonable to skip or limit the extent of the EDX studies. Finally, EDX studies may be normal in selected muscle diseases (certain endocrine, metabolic, congenital, and mitochondrial myopathies). Thus, in the appropriate clinical context, normal EDX studies do not necessarily rule out the presence of a myopathy.
EDX studies are most useful to diagnose a myopathy when further data are needed to exclude alternative diagnoses, confirm the presence of a muscle disease, and narrow down the differential. The role of EDX studies is summarized in Box 1 . First of all, the results of nerve conduction studies (NCSs) and electromyography (EMG) are used to exclude neuromuscular conditions that may mimic a myopathy (such as motor neuron disease and neuromuscular junction disorders or, occasionally, motor neuropathies). Second, EMG is often able to confirm the diagnosis of a muscle disorder, when motor units with characteristic morphology and recruitment pattern are identified ( Figs. 1 and 2 ). In such cases, EMG may also add diagnostic information relating to the location, type, and severity of the underlying process. For example, the presence of abnormal spontaneous activity may help narrow down the differential among different myopathic processes ( Box 2 ). Finally, EMG may be useful in identifying target muscles for biopsy. This is particularly helpful when the only clinically weak muscles are not easily accessible for biopsy (such as the gluteal muscles, the hip flexors, or the paraspinals). The yield of a muscle biopsy increases when a weak (Medical Research Council grade 4 of 5), but not end-stage, muscle is biopsied. EMG analysis allows the evaluation of multiple sites and the identification of affected muscles that are not weak on neurologic examination.
- 1.
Exclude neuromuscular conditions that may mimic a myopathy
- a.
Motor neuron disease
- b.
Motor neuropathies
- c.
Neuromuscular junction disorders
- a.
- 2.
Provide EMG evidence of the presence of a myopathy (although EMG may be normal in the presence of selected myopathic processes)
- 3.
Characterize the myopathy
- a.
Location (proximal, distal, symmetric, or asymmetric)
- b.
Presence/absence of abnormal spontaneous activity
- c.
Severity
- a.
- 4.
Identify target muscles for biopsy
- 1.
Inflammatory myopathies (often)
- a.
Polymyositis
- b.
Dermatomyositis
- c.
Inclusion body myositis (IBM)
- d.
Immune-mediated necrotizing myopathy (with or without association with cholesterol-lowering agents)
- a.
- 2.
Toxic/necrotic myopathies (often)
- a.
Cholesterol-lowering agent myopathies (eg, statin myopathies)
- b.
Critical illness myopathy
- c.
Chloroquine/hydroxychloroquine
- d.
Amiodarone
- e.
Colchicine
- a.
- 3.
Muscular dystrophies, including the distal muscular dystrophies/myopathies, hereditary inclusion body myopathies, and the myofibrillar myopathies
- 4.
Congenital myopathies (some)
- a.
Nemaline rod myopathy
- b.
Centronuclear/myotubular myopathy
- c.
Central core myopathy
- d.
Multicore/minicore myopathy
- a.
- 5.
Myopathies associated with selected infectious agents
- a.
HIV and human T-lymphotropic virus 1–associated myositis
- b.
Trichinosis
- c.
Toxoplasmosis
- a.
- 6.
Metabolic myopathies (some)
- a.
GDS II (acid alpha-glucosidase deficiency or Pompe disease)
- b.
GSD III (debrancher enzyme deficiency)
- c.
GSD IV (branching enzyme deficiency)
- d.
Lipid storage myopathies
- a.
- 7.
Myotonic disorders
- a.
Myotonic dystrophy type 1 (DM1) and myotonic dystrophy type 2 (DM2)
- b.
Myotonia congenita, paramyotonia congenita (PMC), potassium-aggravated myotonias
- c.
Hyperkalemic periodic paralysis
- a.
Electrodiagnostic approach
A practical EDX approach for patients with suspected myopathy is outlined in Box 3 (adapted from other sources).







