Echocardiography and Electrocardiography
James C. Huhta
ECHOCARDIOGRAPHY
Since the early 1980s, echocardiography (i.e., ultrasonic imaging) of the heart and cardiovascular system has provided a major advance in pediatric cardiology and has allowed imaging of anatomy, appraisal of ventricular function, and assessment of peripheral blood velocities in both arteries and veins. This noninvasive modality has altered the assessment of fetal and neonatal congenital heart disease. Early in pediatric cardiology, the electrocardiogram (ECG) was the major tool for the exploration of the intracardiac anatomy, whereas the chest radiograph was the screening tool used for signs of congestive heart failure and for extracardiac anatomy such as the pulmonary artery size and vascularity. No assessment could be made of the fetus because the fetus ECG had not, and still has not, been developed. Now, a complete anatomic and physiologic assessment can be obtained of the neonate and of the fetus at 20 weeks’ gestation.
The technique has changed the practice of pediatric cardiology slowly by replacing cardiac catheterization for the diagnosis of congenital malformations, and combined with the use of prostaglandin for maintaining the patency of the ductus arteriosus, echocardiography has dramatically reduced the need for emergency cardiac catheterization in neonates. Most patients with congenital heart disease detected in the neonatal period can have palliative surgery without cardiac catheterization, and most definitive surgical repairs can be performed successfully without the risks of invasive studies. Pulsed, continuous-wave, color, and tissue Doppler studies have added important capabilities for anatomic and functional assessment. The intraoperative and postoperative management of congenital heart defects has been aided by the addition of transesophageal echocardiography (TEE). This mode can add improved resolution in neonates and older patients for whom performing transthoracic imaging is difficult, and it greatly aids the surgeon by providing feedback about the quality of the repair before separating the patient from the heart-lung machine. Higher-resolution imaging systems continue to evolve and lead to improvements in echocardiography equipment. Made possible by new multielement transducer technology and high-speed computer advances, three-dimensional real-time imaging, color Doppler, and, today, tissue Doppler techniques can be used for an advanced approach to the assessment of systolic and diastolic function of the myocardium.
Diagnosis of Congenital Heart Disease
Echocardiography will continue to be the mainstay of the diagnosis of congenital heart disease in neonates, infants, and children and will become more important as additional experience is accumulated. Pioneered by Van Praagh in 1972 for describing congenital cardiac defects at the autopsy table, the segmental approach has been applied to cardiovascular diagnosis using various methods, including echocardiography. A logical analysis of cardiovascular anatomy requires a step-by-step segmental approach. This segmental approach is used for assessing complex congenital malformations in which some portions of the heart may be absent or malpositioned and for the angiographic delineation of cardiac anatomy. The segmental approach is based on the condition that all aspects of abnormal cardiovascular morphology can be broken down into discrete, mutually exclusive descriptors, allowing any complex congenital malformation to be described unambiguously. The schema must include information on the presence, position, and connection of each cardiac segment. Classically, three segments have been recognized: atria, ventricles, and great arteries. In complex malformations, a detailed approach to segmental diagnosis that can be used for noninvasive examination in any setting is necessary.
By describing the anatomic segments and indicating the normality or abnormality of each, a complete description of the cardiac anatomy can be made. Data are obtained by combining the findings from several echocardiographic windows. A complete, step-by-step approach to cardiac diagnosis includes atrial situs diagnosis; identification of the chambers and their interconnections; and a systematic assessment of valves, septa, coronary arteries, systemic and pulmonary veins, and aortic anatomy. Coding of cardiac anatomic abnormalities now has been implemented by the collaboration of many surgical and cardiology groups.
Cardiac Function Assessment
Echocardiography is a tomographic anatomic tool, but it also provides dynamic information about cardiac function and structure. Doppler echocardiography can provide functional information that is not available from any other method. For example, atrioventricular (AV) valve regurgitation can be diagnosed, and the severity, which depends on many technical and physiologic factors, can be estimated. In addition to seeing a ventricular septal defect, the jet of a left-to-right shunt can be detected by pulsed Doppler, the pressure gradient quantitated by continuous-wave Doppler (using the simplified Bernoulli equation: pressure gradient = four times the peak velocity squared), and the defect spatially oriented by color Doppler. Using the continuity equation and the PISA concept, the flow area and volume can be calculated in left-to-right shunts, and the regurgitant volume and area in AV valve regurgitation can be calculated. The pulmonary artery pressure can be estimated using the peak velocity of the tricuspid regurgitation jet, and the severity of semilunar stenosis or coarctation can be estimated using the peak and mean gradients. These hemodynamic findings can be integrated into this segmental approach using the anatomic segment as the finding and the functional aspect as the modifier. For example, the morphologic mitral valve is the anatomic site and location, and the regurgitation is the modifier.
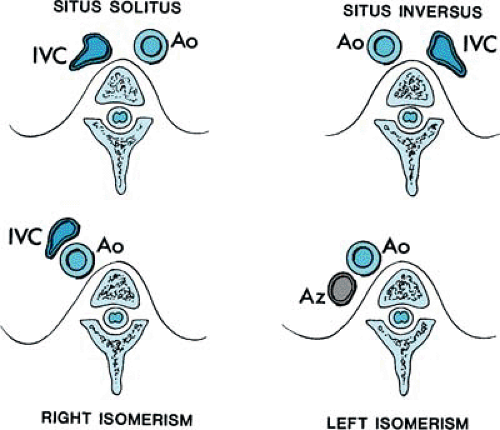
BOX 260.1. Atrial Situs Seen on Echocardiography
Cardiac position and morphology may be described as follows: solitus (normal); inversus; or heterotaxic, with right atrial isomerism or left atrial isomerism. In situs solitus, the morphologic right atrium is on the right, and the morphologic left atrium is on the left. Abnormal atrial situs and cardiac malposition, such as dextrocardia, frequently are associated. Both conditions can be diagnosed by obtaining a short-axis scan of the abdomen, thereby identifying the spine, the inferior vena cava, and the descending aorta. The location of the cardiac apex is important for later scanning from the apex. Subcostal scanning above the diaphragm immediately shows the position of the cardiac apex. From this scan below the diaphragm, the position of the inferior vena cava and aorta usually can be identified, and the location of these structures with respect to the spine identifies the situs (see Fig. 260.1).
The descending aorta and the inferior vena cava are oriented symmetrically with respect to one another, with the inferior vena cava to the right in situs solitus and to the left in situs inversus. In right atrial isomerism, the aorta and the inferior cava run together on either side of the spine, with the cava anterior. A venous structure that courses behind the aorta and does not enter the heart suggests azygos continuation of the inferior vena cava, which is associated with left atrial isomerism. These patients usually have separate, anomalous hepatic venous connections to the heart. Occasionally, atrial appendage morphology can be identified and the diagnosis of atrial situs confirmed directly. A broad-based atrial appendage usually is a morphologic right one, and a narrow-based appendage is morphologic left. Symmetric appendages suggest atrial isomerism. A prospective study of the accuracy of indirect methods showed a sensitivity of 100% and a specificity of 99% for detection of abnormal situs by echocardiography.
Structure-Oriented Approach
Adult-oriented, two-dimensional echocardiographic reports usually take a view approach to the cardiac anatomy, which usually is highly predictable. The investigators describe the appearance of a given cardiac lesion in a standard parasternal, apical, or subcostal scan. However, this approach can lead to diagnostic errors when it is applied to congenital heart disease. For example, a scan of an aortopulmonary window from the right ventricular outflow tract can simulate the origin of the aorta from the right ventricle (i.e., transposition of the great arteries). In congenital heart disease, the various views must be integrated, scanning from one to another echocardiographic window to perform a complete anatomic examination. Although the echocardiographic examiner with experience learns to identify the normal appearances of the heart without congenital defects from various echo windows, a structure-oriented or anatomic approach always is superior to one based on standardized views.
The diagnostic accuracy of two-dimensional echocardiographic imaging in children has been proven in several prospective studies, including that of Gutgesell and colleagues. In 250 consecutive children with congenital heart disease treated at Texas Children’s Hospital, Houston, Texas, only one surgically significant error in diagnosis occurred. This standard requires a compulsive approach to detect rare variations in anatomy that can have importance to the surgeon. For example, coronary artery anomalies such as a coronary originating from the pulmonary artery can be detected using a standardized approach to defining the origins and courses of the coronary branches.
Situs Diagnosis
The diagnosis of cardiac position and atrial-visceral situs using echocardiographic signs is a standard part of the assessment of congenital heart disease and is the foundation of the segmental approach. Atrial situs and atrial morphology are diagnosed together, and possibilities are described in Box 260.1 (Fig. 260.1).
Atrioventricular Connection
The diagnosis of a connection of the atria and ventricles (i.e., AV connection) requires knowledge of the atrial and ventricular morphology. The echocardiographic criteria for a morphologic left ventricle include insertion of the mitral valve at the crux of the heart farther from the cardiac apex than from the tricuspid valve, two normally placed left ventricular papillary muscles, mitral semilunar continuity, a typical elliptic, smooth wall septum, and a fish-mouth appearance of the mitral valve with two commissures. In the absence of the typical offsetting of the AV valves and with cardiac malposition, the trabecular pattern of the ventricles sometimes can be recognized: a smooth wall pattern of the left ventricle and coarser, more heavily trabeculated pattern of the right ventricle. The appearance of the ventricular outflow tracts may aid in establishing ventricular morphologic diagnosis and should be observed as part of the segmental approach. Normally, a continuity between the mitral valve of the left ventricle and the aortic valve is present, but muscle separates the tricuspid and the pulmonary valves in the right ventricular outflow tract. The most reliable criterion for identification of the morphologic right ventricle is tricuspid valve chordal attachments to the septum. With an atrial septal defect of the primum type, the valves are at the same
level (Fig. 260.2). Four types of AV connection are described in Box 260.2.
level (Fig. 260.2). Four types of AV connection are described in Box 260.2.
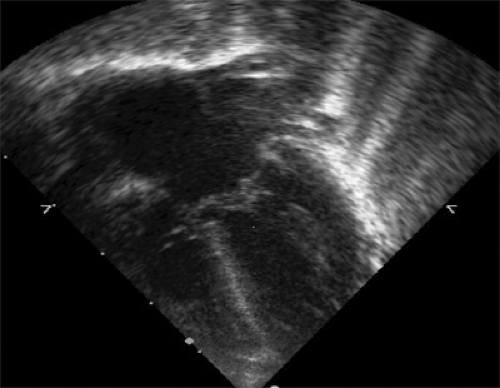 FIGURE 260.2. Atrioventricular (AV) canal defect with a large primum atrial septal defect. Note the mitral and tricuspid valves are at the same level. |
The accuracy of echocardiographic imaging in the diagnosis of AV connection is unsurpassed by other modalities. Occasionally, an inexperienced observer may confuse a common inlet with a common (four-leaflet) valve with a single inlet. After experience with imaging the variations of AV septal defect, this differentiation should not present problems. Identification of the lower atrial septum unequivocally identifies the crux of the heart and points to a single inlet with atresia of the other valve. The general consensus is that echocardiography in experienced hands is the best method for assessing AV connection and for assessing abnormalities of the cardiac valves.
BOX 260.2. Atrioventricular Connections Seen on Echocardiography
Atrioventricular connections may be any of the following: concordant (i.e., normal); discordant; univentricular through a single inlet (i.e., tricuspid or mitral atresia), double inlet, or common inlet; or ambiguous (i.e., two ventricles with atrial isomerism). When the morphologic right atrium connects normally to the morphologic right ventricle and the left atrium connects to the left ventricle, atrioventricular concordance is present. When this connection is reversed and the morphologic right atrium connects to the morphologic left ventricle, atrioventricular discordance is present; this referred to as ventricular inversion. Patients with this abnormality may present with complete heart block and have a high incidence of associated congenital cardiac malformations, such as ventricular septal defect and pulmonary stenosis; they usually also have ventriculoarterial discordance. Rarely, atrioventricular discordance may occur when the ventriculoarterial connection is normal. When most of the atrioventricular connection is to one ventricle, the connection is univentricular through one valve (i.e., single inlet with atresia of the other valve), a double inlet (i.e., two atrioventricular valves), or a common inlet (i.e., common atrioventricular valve). A common inlet ventricle is part of the spectrum of atrioventricular septal defect (i.e., atrioventricular canal) in which hypoplasia of one of the ventricular chambers occurs and the atrioventricular connection is predominantly to the other.
BOX 260.3. Types of Ventriculoarterial Connections Seen on Echocardiography
Ventriculoarterial connections may be concordant (i.e., normal), discordant (i.e., right ventricle to the aorta and left ventricle to the pulmonary trunk), double outlet (usually the right ventricle), or single outlet (i.e., aortic or pulmonary atresia or truncus arteriosus). When the morphologic right ventricle connects to the pulmonary valve and the morphologic left ventricle connects to the aortic valve, concordance is present. When the morphologic right ventricle gives rise to the aorta and the morphologic left ventricle gives rise to the pulmonary trunk (i.e., ventriculoarterial discordance), transposition of the great arteries, the most common type of abnormality of ventriculoarterial connection, occurs (see Fig. 260.3). To diagnose this abnormality, the great vessels must be identified. The pulmonary artery is identified by its branching pattern into left and right pulmonary arteries and ductus arteriosus, and the aorta is identified by the coronary, carotid, and subclavian arteries. Both great vessels may originate from either ventricle (usually the morphologic right ventricle), creating a double-outlet right ventricle. If the aortic or pulmonary valve is atretic, a single-outlet ventricle is the result. When a single truncal valve originates from the ventricular mass but overrides the ventricular septum, truncus arteriosus, another example of a single-outlet abnormality, occurs. The ventriculoarterial connection is designated as a single outlet with an overriding truncal valve. In complex malformations, including right atrial isomerism with the asplenia syndrome, the atrioventricular septal defect often is associated with a double-outlet right ventricle. In tetralogy of Fallot, overriding of the aortic valve often is present so that almost one-half of the valve annulus appears to arise from the right ventricle. Mitral aortic continuity is present, and, except for the rare circumstance in which more than 50% overriding of the aortic valve occurs, the ventriculoarterial connection in tetralogy of Fallot is concordant.
Ventriculoarterial Connection
Ventriculoarterial connection is the manner in which the great arteries and semilunar valves connect to the ventricular outflow tracts. Normally, the morphologic right ventricle connects to the pulmonary valve, and the morphologic left ventricle connects to the aortic valve. Four types of AV connection are described in Box 260.3.
Reports of neonates with abnormalities of ventriculoarterial connection and children with transposition of the great arteries show that echocardiography can detect accurately these abnormalities (Fig. 260.3). A newborn with cyanosis caused by transposition can be diagnosed without catheterization, and most neonates now have surgery without catheterization.
Atrial and Ventricular Septa
Atrial Septum
Before birth, the atrial septum usually bows toward the morphologic left atrium because of the significant
blood flow to the left side of the heart through the fossa ovalis. This aneurysmal bowing of the atrial septum after birth may be a clue to right-to-left or left-to-right intraatrial shunting if it is bowing toward the right atrium. Color Doppler studies have confirmed that left-to-right shunting through a patent foramen ovale is a normal finding soon after birth, particularly if the ductus arteriosus has not closed. After the infant reaches 6 weeks of age, persistent shunting at the atrial level is considered abnormal if the color diameter of the shunt is greater than 3 mm.
blood flow to the left side of the heart through the fossa ovalis. This aneurysmal bowing of the atrial septum after birth may be a clue to right-to-left or left-to-right intraatrial shunting if it is bowing toward the right atrium. Color Doppler studies have confirmed that left-to-right shunting through a patent foramen ovale is a normal finding soon after birth, particularly if the ductus arteriosus has not closed. After the infant reaches 6 weeks of age, persistent shunting at the atrial level is considered abnormal if the color diameter of the shunt is greater than 3 mm.
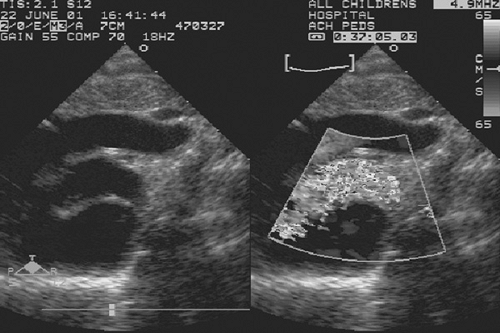 FIGURE 260.3. Transposition of the great arteries (discordant ventriculoarterial connection). Note the parallel exit of the anterior aorta and the posterior pulmonary artery. |
The results of echocardiographic imaging of atrial septal defects are good. Detailed analysis of the venous connections is needed to exclude partial anomalous pulmonary venous return, for example. In the prospective study of Gutgesell and associates, one false-positive and one false-negative result for diagnosing sinus venosus defects occurred. Since then, the triage of patients with an atrial defect requires detailed measurements of the rims of the defect to determine whether the patient is a candidate for the device closure of the defect in the catheterization laboratory. The popular Amplatzer device straddles the hole and effectively closes it permanently. Another practical application of echocardiography is the evaluation of the atrial defect created by balloon atrial septotomy or blade and balloon techniques.
A thin strand of tissue in what appears to be a common atrium suggests right atrial isomerism. The upper atrial septum, where a sinus venosus defect may occur, can be difficult to evaluate in an older child, but color flow mapping has improved the ability to evaluate all forms of atrial septal defect.
Ventricular Septum
Defects of the ventricular septum can be analyzed using multiple tomographic imaging approaches, and defects can be separated into those that are perimembranous, muscular, or subarterial. An inlet perimembranous defect (i.e., AV canal-type defect) can be differentiated from the AV canal by the presence of the central fibrous body at the internal crux of the heart. Small muscular ventricular septal defects and even a significant defect in the perimembranous region may be missed by imaging alone, but color Doppler has improved substantially the ability to detect muscular defects. With multiple ventricular septal defects, color may be crucial for detection. The sensitivity in older children with smaller muscular ventricular septal defects and another large ventricular septal defect was only 72% in one study. The details of complicated interventricular communications in the trabecular septum may be aided by angiography or a detailed evaluation by TEE. Echocardiography with color Doppler appears to be adequate to evaluate these patients by using the transesophageal approach, especially in older patients. Three-dimensional echocardiography offers the promise of improved spatial orientation and delineation of the defect.
Valves
Atrioventricular Valves
A wide variety of malformations may involve the left or right AV valves. The mitral or tricuspid valve may be abnormally positioned, stenotic, regurgitant, or hypoplastic, or the valve may have a cleft or exhibit prolapse, straddling, or Ebstein malformation. The pattern of opening on real-time imaging is augmented by the Doppler or M-mode functional assessment. Virtually all forms of congenital abnormalities of the mitral valve can be recognized immediately by imaging alone, with the possible exception of supravalvar mitral ring, in which the ring may adhere to the valve tissue. The normal papillary muscles in this disorder differentiate it from most other congenital forms of mitral stenosis. Color flow mapping and continuous-wave Doppler more effectively evaluate the hemodynamics of AV valve stenosis than do invasive techniques. Regurgitation of AV valves can be detected with excellent sensitivity, and color Doppler scans can enable the examiner to grade the severity of regurgitation of the AV and semilunar valves.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree