Double-Outlet Right Ventricle
Michael J. Silka
Double-outlet right ventricle (DORV) refers to a diverse group of congenital heart defects characterized by common origin of both the aorta and the pulmonary artery above the morphologic right ventricle. DORV specifies the anatomic relationship of the great arteries to the right ventricle; however, it does not specify circulatory physiology, which is determined largely by the combination of associated congenital heart defects. Historically, various terms, including Taussig-Bing complex, origin of both great vessels from the right ventricle, and partial transposition, have been applied to this malformation and subtypes.
DORV is an uncommon finding, representing 1.5% of all congenital heart defects, with a predicted incidence of 1 case per 15,000 live births. Although associations with trisomy 18 and maternal diabetes are recognized, most cases of DORV occur in infants with no other congenital anomalies. The embryologic basis of DORV involves failure of rotation and shift of the truncus arteriosus from above the primitive right ventricle (bulbus cordis) to a more leftward position, which aligns the future aorta above the morphologic left ventricle. Varying degrees of arrest of conoventricular rotation and leftward truncal shift result in the spectrum of congenital heart defects grouped as DORV.
ANATOMY AND PHYSIOLOGY
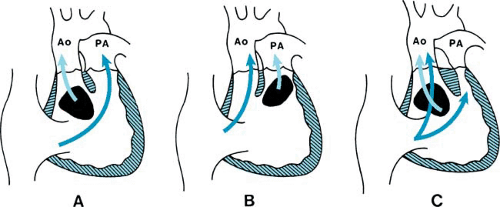
DORV is a specific malformation representing one of the types of malposition of the great arteries. A ventricular septal defect (VSD), which provides the only outlet for the left ventricle, is located beneath either the pulmonary artery or the aorta or, rarely, remote from both great arteries (Fig. 266.1). Associated subpulmonic stenosis, which restricts pulmonary blood flow, is present in more than 50% of patients with DORV.
The relationship of the VSD to the great arteries and the presence or absence of pulmonic stenosis are the primary determinants of the circulatory physiology in DORV. When the VSD is related closely to the aorta (see Fig. 266.1A), the physiologic features are similar to those of a large VSD with left-to-right shunt and pulmonary hypertension. In this setting, symptoms of congestive heart failure dominate after the anticipated decrease of pulmonary vascular resistance occurs, with little or no cyanosis. Conversely, when the pulmonary artery overrides the VSD, without associated pulmonic stenosis (see Fig. 266.1B), the hemodynamics simulate those of transposition of the great arteries with a large VSD. Frequently called the Taussig-Bing complex, clinical features of this condition include both cyanosis and congestive heart failure. Coarctation of the aorta and variable degrees of hypoplasia of the subaortic outflow tract often occur in this form of DORV. In either of these subsets of DORV, the clinical course is determined by pulmonary vascular resistance and the presence or absence of left heart obstructive lesions. Severe subpulmonic or pulmonary valvar stenosis occurs most frequently when the VSD is subaortic (see Fig. 266.1C). The resultant physiology and clinical manifestations are indistinguishable from tetralogy of Fallot, with cyanosis and pulmonary oligemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree