Defects of the Atrial Septum, Including the Atrioventricular Canal
G. Wesley Vick III
Louis I. Bezold
Congenital defects of the atrial septum are common occurrences. They may be located in different anatomic portions of the atrial septum, and the location of the defect generally reflects the abnormality of embryogenesis that led to the anomaly (Fig. 271.1) Atrial septal defect (ASD) may be isolated or may be associated with other congenital cardiac abnormalities. ASD sizes vary greatly, and functional consequences are related to the anatomic location of the defect, the size of the defect, and the presence or absence of other cardiac anomalies.
Developmental defects resulting from abnormalities in partitioning of the embryologic atrioventricular (AV) canal and in the endocardial cushions often lead to a communication between the right and left atria. Most AV canal (endocardial cushion) malformations demonstrate major anomalies of the AV valves in addition to an intraatrial communication. Defects of the ventricular septum often are present.
Interatrial communications can be considered in two groups. The first group results from abnormal development of the septa that normally partition the atrial portion of the developing heart into right and left atria. The second group includes interatrial communications that result primarily from maldevelopment of the partitioning of the AV canal and endocardial cushions. The first group may be viewed as isolated ASDs and the second group as AV canal defects.
ISOLATED ATRIAL SEPTAL DEFECTS
Isolated ASDs include patent foramen ovale, ASD at the fossa ovalis (secundum ASD), defects superior to the fossa ovalis (superior sinus venosus type ASD, superior vena caval defect), defects inferior to the fossa ovalis (inferior sinus venosus type ASD, inferior vena caval defect), and coronary sinus defects. AV canal defects include complete forms, incomplete forms, and common atrium. Pathophysiologic features of ASDs are described in Box 271.1.
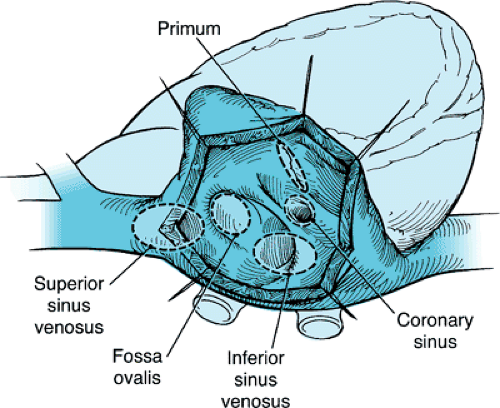 FIGURE 271.1. Atrial septal defects. Only defects within the fossa ovalis are true defects of the interatrial septum, although all the defects permit interatrial shunting. |
Associated Cardiac Defects
ASDs often occur in conjunction with other congenital cardiac anomalies. In many of these anomalies, the associated defects are the lesions of primary importance; however, the ASD may play a major role in the physiologic features of the condition. For example, in complete transposition of the great arteries, an ASD permits mixing between the pulmonary and systemic circulations necessary to sustain life. Another example is tricuspid atresia, in which the entire cardiac output must pass across the ASD.
Effect on Intrauterine and Postnatal Cardiac Physiology
Because pulmonary blood flow is relatively minimal before birth, nearly all blood reaching the left atrium does so by passage across the foramen ovale. When the lungs expand after birth, pulmonary venous return to the left atrium increases substantially, concomitant with a fall in pulmonary vascular resistance and an increase in systemic vascular resistance. As a result, left atrial pressure normally rises above the right atrial pressure, and functional closure of the foramen ovale occurs. When an ASD is present, intrauterine physiology is largely unchanged. However, after birth, the normal hemodynamic changes result in left-to-right shunting at the atrial level. In some patients with elevated pulmonary pressures, right-to-left shunting via the ASD also may occur and may be associated with mild cyanosis in the neonatal period.
Significance of Atrial Septal Defects
Small Defects
Small ASDs are defined as those with a pulmonary-to-systemic flow ratio ([Q with dot above]p:[Q with dot above]s) of less than 2:1 in the absence of significant associated cardiovascular anomalies. The presence of a small ASD does not cause major changes in cardiac hemodynamics, although these defects may allow paradoxical embolism whenever the right atrial pressure rises above the left atrial pressure.
BOX 271.1. Pathophysiologic Features of Atrial Septal Defects
Patent Foramen Ovale
If the flap valve of the foramen ovale (the remnant of the embryonic septum primum) is competent, shunting at the atrial level cannot occur as long as left atrial pressure remains higher than right atrial pressure. However, even in physiologically normal individuals, the right atrial pressure may rise transiently above the left atrial pressure. In this circumstance, right-to-left interatrial shunting may occur if the valve of the foramen ovale is not sealed anatomically or is insufficient to close the foramen ovale. Similarly, blood clots or other emboli may cross the atrial septum from right to left in such circumstances (paradoxical embolism). In patients with pulmonary vascular disease or pulmonary stenosis, right-to-left shunting across a patent foramen ovale may be sufficient to cause systemic arterial desaturation.
Approximately 30% to 40% of normal adult hearts have a patent, valve-incompetent foramen ovale that usually is not considered a true atrial septal defect. This valve incompetence may be congenital or may be acquired by stretching of the right or left atrium in conditions in which those chambers are enlarged.
Defects at the Fossa Ovalis (Secundum Defects)
Typical defects in the fossa ovalis are contained within the area bordered by the limbus of the fossa ovalis. Sizes of these defects vary greatly. In addition, the floor of the fossa ovalis (valve of foramen ovale) in this region may be fenestrated, so multiple defects can occur. Secundum defects may be associated with or confluent with other defects of the atrial septum, such as a sinus venosus defect or an ostium primum defect.
Sinus Venosus Defects
Superior sinus venosus defects (sometimes called superior vena caval defects) are located in the part of the atrial septum immediately below the superior vena caval orifice. The right upper and middle-lobe pulmonary veins often connect to the superior vena caval and right atrial junction, resulting in a partial anomalous pulmonary venous connection.
Inferior sinus venosus defects (sometimes called inferior vena caval defects), which are less common than superior defects, are located in the part of the atrial septum immediately above the inferior vena caval orifice. Frequently, inferior sinus venosus defects also are associated with partial anomalous connection of the right pulmonary veins.
Coronary Sinus Defect
Coronary sinus defects are characterized by absence of part or all of the common wall between the coronary sinus and the left atrium. A persistent left superior vena cava also is present in many cases.
Moderate and Large Defects
Moderate and large ASDs are defined as those associated with a [Q with dot above]p:[Q with dot above]s greater than 2:1 in the absence of significant associated cardiovascular anomalies. The direction of the atrial level shunt is determined by the relative pressures in the right and left atria. The atrial pressures are determined principally by the resistances to filling of the respective ventricles. Thus, with
large defects, the volume of the shunting does not depend solely on the size of the defect, but rather on the relative compliance of the right and left ventricles.
large defects, the volume of the shunting does not depend solely on the size of the defect, but rather on the relative compliance of the right and left ventricles.
Natural History
Isolated secundum ASDs typically do not cause major symptoms during infancy and childhood. In the absence of unrelated problems, more than 99% of patients with isolated secundum defects will live beyond the first year of life. Although unusual, mild cyanosis sometimes is evident during the neonatal period. Children and infants with these defects tend to be somewhat smaller than normal, but failure to thrive on the basis of an ASD alone is a rare occurrence. Exercise intolerance may develop in some patients as early as the second decade of life, whereas others may remain asymptomatic for several more decades.
Left-to-right shunting tends to increase with age in many patients. Thus, the frequency of congestive heart failure with attendant fluid retention, hepatomegaly, and elevated jugular venous pressure increases with the age of the patient. The large shunts present in many older patients cause stretching of the atria, which presumably predisposes to atrial arrhythmias, including atrial flutter, fibrillation, and tachycardia. These arrhythmias are a major cause of morbidity and mortality in older patients with ASDs. Pulmonary vascular disease does develop occasionally in older patients with isolated ASD, but this complication is a rare finding in children and adolescents.
A recent natural history study on the growth of secundum ASDs reported growth in the size of the defect in 65% of children at a mean rate of 0.8 mm/year. Although defects greater than 12 mm in diameter tended to have a higher rate of growth than did smaller defects, this study demonstrated the potential for even initially small defects (less than 6 mm) to enlarge to the point that transcatheter closure may be difficult (larger than 20 mm).
Conversely, what has become apparent is that a substantial number of ASDs detected during infancy will close spontaneously. Radzik and colleagues observed a closure rate of 87% in 101 patients with secundum ASDs identified before 3 months of age using two-dimensional and Doppler echocardiography over a follow-up period of 265±190 days. All defects less than 3 mm (n = 32) closed spontaneously; however, no defect larger than 8 mm (n = 4) was observed to close spontaneously in this study. Spontaneous closure of an ASD also has been documented by cardiac catheterization. Cockerham and colleagues detected the presence of an isolated secundum ASD by cardiac catheterization and cineangiography in 54 patients younger than 2 years of age. Of these patients, 14 were documented to have complete closure of their ASD at a subsequent cardiac catheterization. Other investigators also have described spontaneous complete closure of ASDs in early childhood.
Thus, either enlargement or spontaneous closure of ASDs may occur with time. The substantial rate of spontaneous closure in the first 2 years of life suggests that performing secundum ASD closure is not warranted in infants and young children in the absence of substantial symptoms. The propensity of some defects to enlarge supports the common practice of electively closing moderate size defects in childhood, before they have a chance to enlarge beyond the size at which performing transcatheter closure is feasible.
Pregnancy
Pregnancy places additional demands on the cardiovascular system and may cause patients with previously occult ASDs to become symptomatic. In particular, exercise intolerance and congestive heart failure may become apparent during pregnancy. Venous thrombosis secondary to venous stasis in pregnancy may lead to paradoxical embolism in the presence of an ASD. When pulmonary vascular disease is present, pregnancy carries a substantial health risk for the mother and often results in miscarriage.
Diagnosis
Physical Examination
Inspection and Palpation
As mentioned earlier, children with ASDs may be slightly smaller than normal, but failure to thrive is rare. A precordial bulge may be present in those with a large left-to-right shunt, and Harrison grooves (transverse depressions along the sixth and seventh costal cartilages at the site of attachment of the anterior part of the diaphragm) may be apparent. The presence of a hypoplastic thumb, radius, or phocomelia should cause suspicion of Holt-Oram syndrome, an autosomal dominant disorder in which an upper limb deformity is found with congenital heart disease (most often an ASD in association with prolonged AV conduction). Cyanosis may be present in infants, particularly those with right ventricular outflow obstruction or elevated pulmonary artery pressures.
In patients with a thin body habitus and a large atrial left-to-right shunt, a hyperdynamic right ventricular impulse may be observed. Palpation along the left sternal border and in the subxiphoid area will demonstrate this impulse, often termed a right ventricular heave. When pulmonary vascular disease or obstruction to right ventricular outflow exists, the right ventricular impulse is less dynamic and has more of a tapping or thrusting quality, suggesting the presence primarily of pressure rather than volume overload. An enlarged and pulsatile pulmonary trunk may be palpated at the second left intercostal space in many patients and is even more prominent and may be associated with a palpable second heart sound if pulmonary hypertension is present.
Arterial Pulse
The arterial pulse is normal at rest in patients with uncomplicated ASDs. In large ASDs, palpation of the arterial pulse during the straining phase of a Valsalva maneuver may fail to demonstrate the usual pulse amplitude decrease because the large volume of blood pooled in the lungs permits left ventricular output to be maintained.
Jugular Venous Pulse
Patients with isolated and nonrestrictive ASDs have a normal amplitude jugular venous pulse. However, as the two atria are connected by a nonrestrictive channel, the A and V waves of the venous pulse attain equal height. When pulmonary vascular disease supervenes, the right atrium contracts more forcefully, causing large A waves to form.
Auscultation
In patients with ASDs, the first heart sound—best heard at the apex and lower left sternal edge—may be single or split with an intensified second (tricuspid) component. The classic characteristic auscultatory finding in ASD is wide, fixed splitting of the second sound. This finding is present in patients with large left-to-right shunts and normal pulmonary artery pressure. Additional details about this split second heart sound are presented in Box 271.2.
ASDs with moderate to large left-to-right shunts are associated with a pulmonary systolic ejection murmur that begins shortly after the first heart sound, peaks in early to mid systole, and ends before the second heart sound. A palpable thrill is usually absent and when present suggests either a very large shunt or pulmonic stenosis. Rapid flow through the peripheral pulmonary arteries may cause systolic crescendo-decrescendo murmurs that are most prominent in the peripheral lung fields rather than the second intercostal space. Because the pressure gradient across the atrial septum seldom is large, audible
murmurs from flow across the ASD itself are rare findings (although intracardiac phonocardiography can demonstrate them).
murmurs from flow across the ASD itself are rare findings (although intracardiac phonocardiography can demonstrate them).
BOX 271.2. Second Heart Sound in Patients with Atrial Septal Defects
The fixed splitting of the second sound results from a combination of factors. In physiologically normal individuals, inspiratory splitting of the second sound results primarily from increased pulmonary capacitance during inspiration. With expiration in normal persons, pulmonary capacitance decreases, and splitting of the second sound decreases. In contrast, in patients with atrial septal defects (ASDs) and significant left-to-right shunts, the capacitance of the pulmonary bed is increased, resulting in wide splitting between the first and second components of the second heart sound. Because of less respiratory variation in the pulmonary capacitance, little variation in splitting of the second sound occurs in patients with an ASD. When the left-to-right shunt is small or negligible, as it is in most neonates with ASDs, fixed splitting of the second sound does not occur. Because relatively wide (but not truly fixed) splitting of the second sound occurs commonly in supine patients, evaluation of the second sound is better performed with the patient sitting or standing. The intensities of the pulmonic and aortic components of the second sound are equal in most patients with uncomplicated ASDs. Patients with pulmonary hypertension have an accentuated pulmonic component of the second sound. Occasionally, in a patient with normal pulmonary pressures, the pulmonic component of the second sound is intensified because of the proximity of the dilated pulmonary artery to the chest wall.
The diastolic murmur most commonly associated with ASD is a middiastolic murmur resulting from the high flow across the tricuspid valve. This murmur becomes apparent when the left-to-right shunt is greater than 2:1. The murmur is of low to medium frequency and does not increase with inspiration. Another diastolic murmur sometimes associated with ASD is a low-pitched murmur of pulmonic regurgitation, probably a consequence of dilatation of the pulmonary artery.
In the occasional patient with ASD and right-to-left shunting caused by pulmonary hypertension, auscultatory findings differ greatly from those usually found in ASDs. A right ventricular fourth heart sound may be present. The midsystolic pulmonic murmur is softer and shorter, and the tricuspid diastolic flow murmur is absent as a result of the relatively normal right ventricular stroke volume because of lack of significant left-to-right atrial shunting. The pulmonic component of the second sound is increased in intensity, but the fixed splitting characteristic of ASD is not present. If a murmur of pulmonic insufficiency is present, it is high pitched. A holosystolic murmur of tricuspid insufficiency also may be present.
Electrocardiography
Sinus rhythm is customary in young patients with uncomplicated secundum ASDs. Prolongation of the PR interval is a common finding and sometimes has a familial association. Beyond the third decade of life, patients with ASD have a high frequency of atrial arrhythmias, particularly atrial fibrillation, but including atrial flutter and supraventricular tachycardia.
Patients with secundum ASDs usually have normal P waves. Often, sinus venosus ASDs are associated with a leftward frontal plane P-wave axis (i.e., negative in leads III and aVF and positive in lead aVL), usually caused by an ectopic pacemaker that occurs near the usual anatomic location of the sinus node.
The QRS complex in patients with secundum ASDs often has a slightly prolonged duration and a characteristic rSr′ or rsR′ pattern, thought to result from disproportionate thickening of the right ventricular outflow tract, which is the last portion of the ventricle to depolarize. Often, the term incomplete right bundle branch block is used to describe this QRS pattern, but that term is a misnomer because the pattern is a consequence of hypertrophy and not a conduction disturbance. With increasing degrees of pulmonary hypertension, patients with secundum ASDs tend to lose the rSr′ pattern in lead V1 and develop a tall monophasic R wave with a deeply inverted T wave. Right axis deviation of the frontal plane QRS axis (ranging from +95 degrees to +135 degrees) is often present. A northwest axis or left axis deviation of the QRS axis suggests the presence of an AV canal defect, but such deviation can occur with uncomplicated secundum ASD as well.
Chest Radiography
The chest radiograph in patients with secundum ASD and sizable left-to-right shunts characteristically shows cardiac enlargement (particularly the right atrial shadow) and increased pulmonary vascularity (Fig. 271.2). Typically, increased pulmonary vascularity extends to the periphery of the lung fields, with a dilated pulmonary trunk and central branches. Enlargement of the pulmonary arteries prevents the aortic shadow from forming the border of the cardiac silhouette creating a characteristic “triangular” cardiac shape. Right atrial and right ventricular enlargement are usually present, but the left atrium and ventricle are typically normal size.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







