Essentials of Diagnosis
- Presents with nonspecific symptoms such as cough and dyspnea.
- Associated most often with rheumatoid arthritis, scleroderma, primary Sjögren syndrome, dermatomyositis, polymyositis, and mixed connective tissue disease; rarely with systemic lupus erythematosus.
- High-resolution computed tomography (HRCT) with thin-section images is the test of choice.
- Nonspecific interstitial pneumonia is the most common histopathologic pattern.
- Treatment with prednisone or immunomodulatory therapy (cyclophosphamide, mycophenolate mofetil, azathioprine) or both can be effective.
General Considerations
Interstitial lung disease (ILD) is a common pulmonary manifestation of systemic rheumatic diseases, including rheumatoid arthritis, scleroderma, the inflammatory myopathies, primary Sjögren syndrome, and mixed connective tissue disease. Collectively, these conditions are called connective tissue disease–associated ILD. Symptoms are nonspecific and include cough and dyspnea. ILD can be a significant cause of morbidity and mortality in this patient population, and early diagnosis is essential to help prevent progression of symptoms and decline in pulmonary function.
ILDs are generally categorized based on the underlying histopathologic pattern seen on surgical lung biopsy (Table 64–1). Patients with rheumatic diseases can manifest many of the histopathologic patterns seen in idiopathic cases of ILD, including usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia, and lymphocytic interstitial pneumonia (LIP) (Table 64–2).
| ILD Pattern | Histopathologic Features | HRCT Features |
|---|---|---|
| Nonspecific interstitial pneumonia |
|
|
| Usual interstitial pneumonia |
|
|
| Organizing pneumonia |
|
|
| Lymphocytic interstitial pneumonia |
|
|
| Diffuse alveolar damage |
| Diffuse ground-glass attenuation and consolidation |
| Histopathologic Pattern | |||||
|---|---|---|---|---|---|
| Rheumatic Disease | Nonspecific Interstitial Pneumonia | Usual Interstitial Pneumonia | Organizing Pneumonia | Lymphocytic Interstitial Pneumonia | Diffuse Alveolar Damage |
| Inflammatory myopathies | ++ | + | + | + | |
| Mixed connective tissue disease | ++ | + | + | ||
| Rheumatoid arthritis | ++ | ++ | + | + | |
| Sjögren syndrome | ++ | + | + | + | |
| Systemic lupus erythematosus | + | + | + | + | |
| Scleroderma | ++ | + | |||
| Undifferentiated connective tissue disease | ++ | ||||
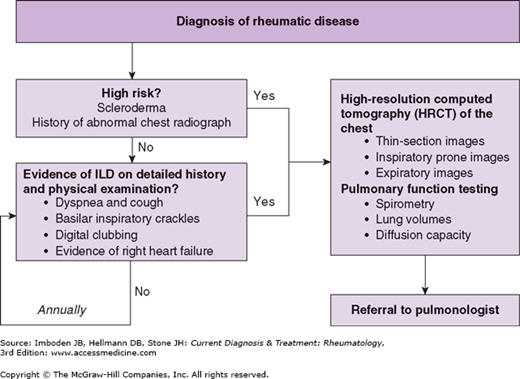
Although the diagnosis of connective tissue disease-associated ILD typically occurs after that of the rheumatic disease, ILD will present first in a certain proportion of patients. Thus, it is not only important to screen rheumatic disease patients for ILD, but the converse as well. Since there is no consensus approach to screening patients for the presence of ILD, obtaining a careful history and performing a physical examination targeting ILD is recommended in all patients with rheumatic disease. More specific testing should be reserved for those patients with signs or symptoms concerning for ILD or who are at high risk for its development (eg, scleroderma). A schematic approach to screening rheumatic patients for ILD is presented in Figure 64–1.
Clinical Findings
The clinician should screen patients for ILD by obtaining a detailed history and performing a physical examination. Patients with ILD often report symptoms of chronic dyspnea and cough as well as a change in exercise tolerance. Screening for occupational and environmental exposures, smoking history, and medications that could lead to pulmonary disease should also be performed. On physical examination, pulmonary auscultation may reveal inspiratory crackles, particularly in a basilar distribution. In addition, digital clubbing may be seen. Given that pulmonary hypertension is often seen in association with ILD, the clinician should also evaluate the patient for signs of right-sided heart failure, including an elevated jugular venous pressure and peripheral edema (see Chapter 63).
The chest radiograph is an inexpensive but insensitive way to evaluate for ILD. Affected patients may have a chest radiograph that demonstrates a bibasilar reticular pattern. However, because the chest radiograph lacks sensitivity, particularly in early disease, it is recommended that an HRCT scan be performed in any patient with suspected ILD.
Advances in radiologic imaging have enhanced the ability to evaluate for ILD, especially with HRCT scans. Individual imaging centers may vary in how the HRCT protocol is performed. The recommended approach is to order an HRCT scan with 1-1.5 mm collimation (thin-section images) at 1-cm intervals. Prone imaging should be performed because atelectasis can develop in the dependent regions of the lung (ie, the posterior basal portions when supine) and mimic ILD. Lastly, air trapping is an important indicator of airways disease, and therefore expiratory imaging should also be included.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree