Chapter 79 Coenzyme Q10
 Introduction
Introduction
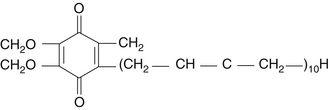
Coenzyme Q10 (CoQ10) is an endogenous proenzyme synthesized naturally in the human body.1 Because of its ubiquitous presence in nature and its quinone structure, CoQ10 is also known as ubiquinone (Figure 79-1). The two major physiologic actions of CoQ10 are (1) as a cofactor in the production of adenosine triphosphate (ATP), and (2) as an antioxidant. Because most cellular functions depend on an adequate supply of ATP, CoQ10 is essential for the health of virtually all human tissues and organs. Cellularly, the highest concentration of CoQ10 is found in the inner mitochondrial membrane, where it facilitates energy production, but CoQ10 is found in the cell membranes of many organelles where it plays a role in membrane stability.2 CoQ10 is the only endogenously synthesized lipid soluble antioxidant.3 In its role in electron transport, the CoQ10 molecule continuously goes through an oxidation-reduction cycle. As it accepts electrons, it becomes reduced to ubiquinol. As it gives up electrons, it becomes oxidized to ubiquinone. In contrast to other antioxidants, this compound inhibits both the initiation and the propagation of lipid and protein oxidation. In its reduced form, ubiquinol, the CoQ10 molecule holds electrons rather loosely, so the CoQ10 molecule will quite easily give up one or both electrons and, thus, act as an antioxidant. It is especially protective against the oxidation of bases of mitochondrial DNA. In addition, ubiquinol is responsible for regenerating vitamin E from the α-tocopheroxyl radical and, thereby interfering with the propagation step of lipid peroxidation.
Biosynthesis of CoQ10 starts from acetyl coenzyme A (CoA) and flows through a multistep process of the mevalonate pathway to produce farnesyl-PP, the direct precursor for not only CoQ10, but also cholesterol, dolichol, and isoprenylated proteins. The long isoprenoid side-chain of CoQ10 is synthesized by trans-prenyltransferase, which condenses farnesyl-PP with several molecules of isopentenyl-PP, all in the trans configuration. The next step involves condensation of this polyisoprenoid side-chain with 4-hydroxybenzoate, catalyzed by polyprenyl-4-hydroxybenzoate transferase. Hydroxybenzoate is synthesized from tyrosine or phenylalanine. In addition to their presence in mitochondria, these initial two reactions also occur in the endoplasmic reticulum and peroxisomes, indicating multiple sites of synthesis in human cells. Nonetheless, numerous conditions are now known to arise in which the body’s synthetic capacity is insufficient to meet CoQ10 requirements. Susceptibility to CoQ10 deficiency appears to be greatest in cells that are the most metabolically active, such as the brain and heart. Tissue deficiencies or subnormal serum levels of CoQ10 have been reported to occur in a wide range of medical conditions and decline with advancing age.3
A need for supplemental CoQ10 could theoretically result from the following:
• Impaired CoQ10 synthesis due to nutritional deficiencies
• A genetic or acquired defect in CoQ10 biosynthesis or utilization
• Increased tissue needs resulting from a particular illness
• The requirement to prevent the side effects of medical intervention
Because oral administration of CoQ10 can increase tissue levels, it is possible to correct CoQ10 deficiency and its associated metabolic consequences by supplementation.4
Coenzyme Q10 Deficiency
Inherited CoQ10 deficiency has been associated with five major clinical phenotypes: (1) encephalomyopathy, (2) severe infantile multisystemic disease, (3) cerebellar ataxia, (4) isolated myopathy, and (5) nephrotic syndrome. In a few patients, pathogenic mutations have been identified in genes involved in the biosynthesis of CoQ10 (primary deficiencies) or in genes not directly related to CoQ10 biosynthesis (secondary deficiencies). Respiratory chain defects, reactive oxygen species production, and apoptosis contribute to the pathogenesis of primary CoQ10 deficiencies.5,6
Acquired CoQ10 deficiency is less well established. There are two major factors that lead to deficiency of CoQ10 in humans: reduced biosynthesis, and increased utilization or need by the body. The typical dietary intake of CoQ10 is 3 to 5 mg,7 so dietary lack is probably not a significant contributor to CoQ10 deficiency. As mentioned previously, endogenous synthesis is a multistep process that can be affected by aging, disease status, and various medications. Some chronic disease conditions (cancer, heart disease, etc.) are not only associated with reduced biosynthesis, but also increased demand for CoQ10. Measurements of plasma CoQ10 levels have been used to detect deficiencies and are by far the most common clinical assessment of CoQ10 status.8 Normal plasma levels are believed to range from 0.45 to 1.5 mcg/mL (or 0.46 to 1.78 µmol/L), with 93% to 100% being the reduced form, ubiquinol.8,9 Some studies used a functional assessment using an assay that measures the citric acid cycle (Krebs cycle) enzyme succinate dehydrogenase-CoQ10 reductase.10 CoQ10 acts as a critical coenzyme to this enzyme, so if the enzyme is fully saturated with CoQ10 in vivo, addition of exogenous CoQ10 does not increase enzyme activity. Additional methods for assessing CoQ10 status are also becoming available.5,11,12
Commercial Forms and Dosage Considerations
A very important consideration in the clinical application of CoQ10 is its pharmacokinetics. CoQ10 as ubiquinone is a crystalline powder that is insoluble in water and has poor absorption characteristics as a result. Ubiquinol has greater solubility and has been promoted as having greater bioavailability that ubiquinone,4,13 but there are limited data currently available and many questions remain to be answered, since ubiquinol is easily oxidized to ubiquinone and absorption studies to date have been suspect. Ubiquinone has an extensive history of use, particularly in oil-based soft-gelatin capsules. Several technologies are now used to enhance the bioavailability of ubiquinone, such as particle size reduction (nanonization) and solubility enhancement via use of emulsifying agents, carriers, and self-emulsifying systems.14 For example, complexing ubiquinone to a soy peptide (BioQ10 SA) has shown exceptional bioavailability because the soy peptide emulsifies the CoQ10 and helps usher it into the bloodstream.15 Given the excellent absorption of this form of ubiquinone, the advantage of ubiquinol over regular ubiquinone appears to have more to do with its improved solubility than because it is in the ubiquinol form.
In light of the pharmacokinetic issues, proper clinical dosing is yet to be determined for CoQ10. Therefore, some have suggested that therapeutic targeting will likely be based upon achieving specific plasma CoQ10 levels (e.g., more than 3.5 mg/mL) or tissue saturation.8,16,17 Prescribing a set dosage, even a milligram per kilogram body weight dosage, at this time is a best guess scenario without confirmation by monitoring CoQ10 blood levels, given the differences noted in absorption rates among the different forms as well as the interindividual variability.
Numerous studies have now been conducted claiming enhanced absorption of one form or another.4 However, there are shortcomings in most of these studies, and some of the studies appear to have been set up to show an advantage for commercial reasons. For example, ubiquinol is being promoted as the best absorbed form of CoQ10, yet the published studies were set up in a curious fashion. In the one study examining ubiquinol absorption, the administration of the ubiquinol was always with a total of CoQ10 capsules that included the emulsification agents diglycerol monooleate, canola oil, soy lecithin, and beeswax, and although the study utilized a placebo, it did not compare ubiquinol with ubiquinone.13 It is possible that ubiquinone might have fared just as well if emulsified as well as the ubiquinol in this study. Absorption studies showed that when CoQ10 was given with food, it was absorbed twice as fast and at least two-fold greater than on an empty stomach.18 It is believed that food-induced secretion of bile acids is responsible for the improved absorption.
What is known based on current absorption studies is that eventually a steady state is produced (usually after 3 to 4 weeks of constant dosing), and the absorption of CoQ10 may be limited in some individuals. Dosages that exceed an individual’s absorptive capacity for CoQ10 may have minimal effect on efficacy and unnecessarily increase the cost of treatment. When single dosages of CoQ10 begin to exceed 300 mg, the percentage of CoQ10 absorbed declines. Plasma CoQ10 levels at a dosage of 900 mg/day (as ubiquinone in a oil suspension in a soft gel capsule) are not significantly greater than a 600 mg/day dosage. Divided dosages (e.g., two or three times a day) result in higher plasma levels compared with single dosages, especially at higher dosage levels.
Despite the challenges, based upon existing data from published studies, an attempt can be made to calculate the approximate plasma levels of CoQ10 for different commercial forms at 100 and 300 mg doses for at least 30 days with several caveats. First, these are estimates only, based upon a typical 75 kg body weight. Keep in mind that absorption of CoQ10 in any form is likely enhanced considerably if taken with a large meal that includes some fat. Lastly, considerable pharmacokinetic studies in humans have indicated significant interindividual variability in CoQ10 absorption, underscoring the need for monitoring CoQ10 plasma levels during clinical studies and perhaps clinical use of CoQ10 as well (Table 79-1).
TABLE 79-1 Coenzyme Q10 Plasma Levels
| DOSE AND FORM | ESTIMATED PLASMA LEVELS IN MICROGRAMs PER MILLILITER |
|---|---|
| 100 mg | |
| Ubiquinone powder in hard gelatin capsule | 1.25 |
| Ubiquinone suspended in oil in soft gelatin capsule with rice bran oil | 1.8 |
| Ubiquinone solubilized in soft gelatin capsule | 2.25 |
| Ubiquinone powder nanonized | 2.25 |
| Ubiquinone emulsified with soy peptide in soft or hard gelatin capsule | 2.50 |
| Ubiquinol in soft gel capsule | 2.50 |
| 300 mg | |
| Ubiquinone powder in hard gelatin capsule | 2.5 |
| Ubiquinone suspended in oil in soft gelatin capsule with rice bran oil | 3.5 |
| Ubiquinone solubilized in soft gelatin capsule | 5.0 |
| Ubiquinone powder nanonized | 5.0 |
| Ubiquinone emulsified with soy peptide in soft or hard gelatin capsule | 7.0 |
| Ubiquinol in soft gel capsule | 7.0 |
The optimal dose of CoQ10 for many clinical indications is not known, and plasma or target tissue levels of CoQ10 are likely the primary determinant of efficacy, rather than the dose.16,19 Current opinion based upon the scientific literature is that an acceptable therapeutic plasma target level of CoQ10 should be at least 2.5 mg/mL,20 but levels higher than 3.5 mcg/mL may be necessary for optimum improvement in neurodegenerative diseases and myocardial function.8,16,19 The usual starting dosage for CoQ10 is generally 100 to 200 mg/day. Where possible, the dosage of CoQ10 should be adjusted according to the response of the patient and, preferably, by monitoring plasma CoQ10 levels for 3 to 4 weeks of constant dosing, when steady-state plasma concentrations occur. Less is known about the ability of CoQ10 and the dose required to reach the central nervous system. Again, divided dosages result in higher plasma levels compared with single dosages, e.g., taking 100 mg twice a day produces higher plasma levels compared with taking 200 mg once a day.21
 Clinical Applications
Clinical Applications
General Antioxidant
Numerous studies have shown CoQ10 can reduce oxidative damage, DNA strand damage, LDL oxidation, and formation of lipid peroxides, thereby supporting its use as a general antioxidant.2,22 In particular, CoQ10 is often used to counteract the reduced synthesis of CoQ10 associated with aging. After the age of 35 to 40 years, humans slowly begin to lose their ability to synthesize CoQ10.23 It has been proposed that the increase in age-associated diseases is due in part to decreased protection afforded by CoQ10 as both an antioxidant and a facilitator of energy production at a cellular level. Therefore, COQ10 is often recommended to counteract this effect.
For example, recent studies identified oxidative stress as a promoting factor for dry mouth (xerostomia) and the development of Sjögren’s syndrome, a condition associated with significant dry mouth. Basically, oxidative damage leads to the inability of salivary cells to produce enough ATP to secrete sufficient amounts of water. CoQ10 exerts antioxidant effects, but its main action in relieving dry mouth may be by increasing energy (ATP) production, allowing the saliva producing cells enough energy to secrete more saliva into the mouth. In one double-blind study, 66 patients, including 31 with dry mouth, were given either ubiquinol or ubiquinone orally at a dosage of 100 mg/day, or a placebo for 1 month.24 Salivary secretion and salivary CoQ10 content were analyzed before and after treatment. Among the dry mouth patients treated with ubiquinone, salivary secretion increased significantly from 0.7 g/2 min before treatment to 1.2 g/2 min after 1 month of treatment. Among the patients treated with ubiquinol, salivary secretion also increased significantly from 0.8 g/2 min before treatment to 1.4 g/2 min after treatment. In normal subjects without dry mouth, salivary secretion increased with ubiquinone (from 4.9 to 5.7 g/2 min) at a statistically significant level, but did not differ significantly after treatment with ubiquinol (from 3.5 to 3.8 g/2 min). Either form of CoQ10 exhibited a marked increase in salivary CoQ10 concentration (ubiquinol more than ubiquionine in dry mouth, ubiquinone more than ubiquinol in normal subjects), suggesting that the observed increase in salivary secretion was attributable to the effect on salivary levels of CoQ10.
Cardiovascular Disease—General Considerations
Enhancing myocardial function is an important, although frequently overlooked, component of the overall prevention and treatment of cardiovascular disease. CoQ10 plays a key role in energy production and is therefore essential for all energy-dependent processes, including heart muscle contraction. CoQ10 deficiency has been documented in patients with various types of cardiovascular disease. Whether a decline in CoQ10 levels is a cause or a consequence of heart disease is unclear. Cardiac cells are highly metabolically active, and thus have higher mitochondrial coenzyme requirements to maintain ATP production. In addition to its role in cellular energetics, exogenously administered CoQ10 acts as an antioxidant to inhibit LDL oxidation,25 decreases proinflammatory cytokines interleukin-6 and tumor necrosis factor-α, and attenuates markers of oxidative and nitrative stress in a dose-dependent manner.2
CoQ10 deficiency is common in patients with various types of cardiovascular disease.2,26 Approximately 75% of patients who had cardiac surgery were shown to be deficient in myocardial CoQ10. Concentrations of CoQ10 declined progressively in both blood and myocardial tissue with increasing severity of heart disease.27 Myocardial deficiencies of CoQ10 were also found in the majority of patients with aortic stenosis or insufficiency, mitral stenosis or insufficiency, diabetic cardiomyopathy, tetralogy of Fallot, atrial septal defects, and ventricular septal defects.28
Cardiomyopathy
A prospective, randomized, double-blind, placebo-controlled trial was conducted in 38 children with idiopathic dilated cardiomyopathy. After 6 months of supplementation, there was a statistically significant (P = 0.011) improvement in diastolic function, leading the authors to conclude that “administration of coenzyme Q10 is useful in ameliorating cardiac failure in patients with idiopathic dilated cardiomyopathy.”29
In one study, 126 patients with dilated cardiomyopathy received 100 mg/day of CoQ10 for up to 66 months. After 6 months of treatment, the mean left ventricular ejection fraction (LVEF) increased from 41% to 59% (P <0.001) and remained stable thereafter with continued treatment. After 2 years, 84% of the patients were still alive, and at 5.5 years, 52% were alive.30 These survival rates were considerably better than the published survival statistics of patients given conventional therapy (i.e., 2-year survival rate of 50% for symptomatic cardiomyopathy, and 1-year survival rate of 50% for decompensated cardiomyopathy).
In another study, 88 patients with cardiomyopathy received 100 mg/day of CoQ10 for 1 to 24 months. Significant improvements in at least two of three cardiac parameters (LVEF, cardiac output, and New York Heart Association [NYHA] class) were seen in 75% to 85% of the patients. Approximately 80% of the patients improved to a lower (i.e., more favorable) NYHA functional class.20
In a double-blind, crossover trial, 19 patients with cardiomyopathy received 100 mg/day of CoQ10 or a placebo, each for 12 weeks. Compared with placebo, CoQ10 treatment significantly increased cardiac stroke volume and LVEF. Eighteen patients reported improvement in activity while taking CoQ10.31
Congestive Heart Failure
The potential of CoQ10 as a treatment for congestive heart failure (CHF) was suggested as early as 1967 by Japanese researchers.32 In 1976, these same investigators administered 30 mg/day of CoQ10 to 17 patients with CHF. All of the patients improved, and nine (53%) became asymptomatic after 4 weeks of treatment.33 Numerous studies since have demonstrated that CoQ10 supplementation resulted in an improvement of stroke volume, LVEF, cardiac output, cardiac index and end-diastolic volume index.2,34,35
In the largest multicenter trial completed to date, 2664 patients in NYHA classes II and III CHF received 50 to 150 mg/day of CoQ10 (78% of patients received 100 mg/day of CoQ10). After 3 months of supplementation, the results showed a low incidence of side effects, and the following proportion of patients had improvements in clinical signs and symptoms36:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree