Chondral Injuries in the Knee
F. Alan Barber MD, FACS
Articular cartilage is a smooth, viscoelastic, hypocellular structure that provides a low coefficient of friction.
The treatment of articular cartilage injury in the athlete presents several challenges. Most athletes want to return to full activity as soon as possible.
Articular cartilage injury in the athlete can occur from shear forces associated with an anterior cruciate ligament (ACL) tear or blunt force trauma to the joint surface.
If the articular cartilage is damaged, a defect may develop.
The goal of treatment is to remove any tissue that is creating a problem and, when needed, to replace it with a tissue that not only fills the defect but also integrates well with the adjacent articular cartilage and does not deteriorate over time.
Patients who present with chondral damage report various mechanisms of injury. These mechanisms often include a pivoting, twisting fall; a significant, direct impact on the knee; an ACL tear; or a patellar dislocation.
Patients commonly report the onset of pain localized to one particular compartment or a persistent, dull, aching pain that worsens after activity and may be most noticeable when the patient tries to fall asleep.
The steps for a successful treatment program include effective communication with the patient, making an accurate diagnosis, and communicating the prognosis.
Debridement of Outerbridge Type 3 and Type 4 lesions as a primary treatment is appropriate for smaller lesions, low-demand patients, and incidentally observed lesions with few symptoms. The best results seem to occur in younger patients with symptoms of less than 1 year in duration, a specific history of trauma, no previous surgery, little to no malalignment, and a low body mass index.
Methods to treat defects in the articular cartilage by stimulating the subchondral marrow include penetrating the subchondral bone by drilling or microfracture and abrasion arthroplasty.
Osteochondral autografting transfers a cylindrical plug of osteochondral material, including both normal articular cartilage with viable chondrocytes and the underlying attached viable subchondral bone from a nonarticulating portion of the joint, into an articular cartilage defect.
The implantation of composite fresh cadaveric allografts is another technique to address full-thickness articular cartilage defects in the knee. One significant issue with allografts is that to optimize survival of the transplanted chondrocytes, the grafts should be implanted as soon after harvest as possible.
Autologous chondrocyte implantation (ACI) is designed to address traumatic focal lesions of the weight-bearing surface, primarily of the femoral condyle. The technique attempts to replace a full-thickness articular defect with hyalinelike tissue. It is indicated for localized, symptomatic full-thickness articular cartilage lesions of at least 2 cm in diameter in young patients with good alignment, good stability, and nonarthritic joints. Patients must be able to comply with the rehabilitation protocol.
Articular cartilage injury is a sports-related injury commonly found at arthroscopic surgery. In a series of more than 31,000 arthroscopies, articular cartilage damage was found in 63% (1). The medial femoral condyle and the patellar surface were the most frequently injured sites (2). These lesions seem to be localized and limited in scope, but what they represent for the future of the knee may not be so innocuous.
The treatment of articular cartilage injury in the athlete presents several challenges. Not only does the average athlete wish to return to full activity as quickly as possible, but the assumption that this will happen as a normal course of seeking medical treatment sometimes raises unrealistic expectations. In addition, more individuals currently continue their athletic activities for a longer time, with the result that older patients are now included in the population sustaining articular cartilage damage.
The articular cartilage of the knee has a complex structure and plays a vital role in the normal athletic activity. Its function is to transmit loads uniformly across the joint and provide a smooth, low-friction, gliding surface. The lack of a vascular response and the relative absence of an undifferentiated cell population to respond to injury make damage to the articular cartilage a problem and limit its healing capacity. Localized full-thickness defects and contusions can cause significant symptoms and are especially problematic because of the potential for these lesions to progress. This is compounded by the natural environment of athletic participation in which the knee is subjected to repeated loading and the potential for violent contact with the ground or other participants.
Articular cartilage is a smooth, viscoelastic, hypocellular structure that provides a low coefficient of friction—estimated to be 20% of the friction seen with ice on ice (3)—and the ability to withstand significant recurring compressive loads. It has a large extracellular matrix composed principally of Type II collagen (60% of the dry weight of cartilage) (4,5). The collagen fibers provide form and tensile strength, and water gives it substance by comprising 75% to 80% of the extracellular matrix. The cellular component (chondrocytes) synthesizes and degrades proteoglycans and is the metabolically active portion of this structure.
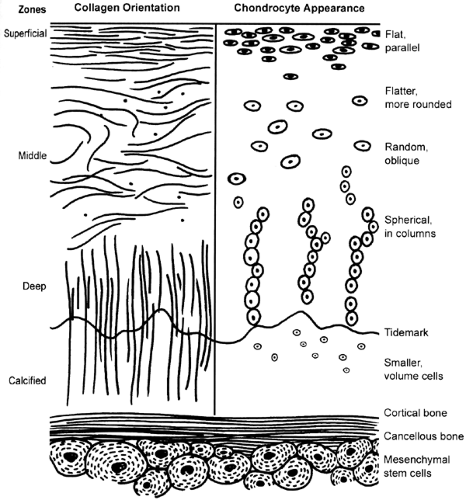
Histologically, articular cartilage has four distinct zones: (a) a superficial (tangential) zone, (b) a middle (transitional) zone, (c) a deep (radial) zone, and (d) the calcified zone (Fig 43.1-1). The primary orientation of the collagen fibers in the superficial zone is parallel with the joint surface to resist compressive and sheer forces. This zone is the thinnest one, and it sometimes is referred to as the gliding zone. The surface layer, known as the lamina splendens, is cellfree and composed mainly of randomly oriented, flat bundles of fine collagen fibrils. Deep to the lamina splendens are more densely packed collagen fibers interspersed with elongated, oval chondrocytes oriented parallel to the articular surface. Little hyaluronic acid and few proteoglycan aggregates, but the highest water concentration, are found in this layer. This superficial zone acts as a barrier by limiting the penetration of large molecules, such as antibodies, into the deeper zone and preventing the loss of molecules from the cartilage into the synovial fluid.
The primary orientation of the middle (transitional) zone collagen fibers is parallel to the plane of joint motion, although some oblique fibers also are present. This layer resists compressive forces, and this zone has more proteoglycans and less water and collagen than the superficial zone. The chondrocytes are more spherical and have more cellular structures, suggesting a matrix synthesis function.
The deep (radial) zone fibers are perpendicular to the surface and resist both compressive and sheer forces. The collagen bundles are arranged in arcades known as the arcades of Benninghoff. These are not continuous (as once thought), and the collagen orientation is somewhat random. In this area, the chondrocytes are round and arranged in columns perpendicular to the joint surface. These cells possess many intracellular structures, and they appear to be very active with protein synthesis. The highest concentration of proteoglycans is found in this zone. The tidemark is located at the base of this zone and also resists sheer stress. It represents a zone of transition from the deep zone to the zone of calcified cartilage. It is an undulating hematoxophilic line, and collagen fibers from the deep zone penetrate this line directly into the calcified cartilage.
The calcified zone acts as an anchor between the articular cartilage and the subchondral bone. The deepest zone, it is a thin layer of calcified cartilage, creating a boundary with the underlying subchondral bone. The cells in this zone usually are smaller and surrounded by a cartilaginous matrix. Damage to any of these layers can alter the normal biomechanical properties of the articular cartilage and lead to progressive deterioration.
The articular cartilage injury that an athlete may sustain can occur from shear forces associated with an ACL tear or from blunt force trauma to the joint surface. This may result in the injury or death of articular chondrocytes. Experimentally, blunt trauma of greater than 25 N/mm2 consistently results in articular chondrocyte death (6). This can play a role in the development of articular cartilage degeneration after injury, but such an injury may not be readily apparent at first.
Areas of chondral injury and subchondral bone edema (i.e., bruising) often are seen on magnetic resonance (MR) images depicting ACL tears. Arthroscopic biopsy of the articular cartilage overlying bone bruises demonstrates superficial articular chondrocyte death, matrix dehydration, and death of the subchondral osteocytes with empty lacuna (7). The inference is that the impact associated with an ACL tear also causes articular cell death in addition to injury to the underlying subchondral bone. The extent and implications of this injury may not be appreciated initially, which may be one explanation for the late appearance of degenerative change after ACL reconstruction.
Areas of chondral injury and subchondral bone edema (i.e., bruising) often are seen on magnetic resonance (MR) images depicting ACL tears. Arthroscopic biopsy of the articular cartilage overlying bone bruises demonstrates superficial articular chondrocyte death, matrix dehydration, and death of the subchondral osteocytes with empty lacuna (7). The inference is that the impact associated with an ACL tear also causes articular cell death in addition to injury to the underlying subchondral bone. The extent and implications of this injury may not be appreciated initially, which may be one explanation for the late appearance of degenerative change after ACL reconstruction.
If the articular cartilage is damaged, a defect may develop. Increased contact pressure is then placed on the edges of the articular cartilage defect and on any exposed subchondral bone. This leads to overloading and degeneration of the defect with an expansion of the lesion. As this situation progresses, the exposed bone contacts the opposing articular cartilage, leading to bipolar injury and, ultimately, a bone-on-bone lesion. The deteriorating cartilage will cause an inflammatory synovitis by the release of breakdown products, and the stress changes of the exposed subchondral bone will lead to venous congestion in the medullary cavity. These may result in chronic aching pain. The goal for the treatment of articular cartilage injury is to remove any tissue that is creating a problem and, when needed, to replace it with a tissue that not only fills the defect but also integrates well with the adjacent articular cartilage and does not deteriorate over time.
One of the challenges faced by the surgeon attempting to meet these goals is the lack of a blood supply or an endogenous source of new cells for the damaged articular cartilage. Spontaneous healing is very limited. Depending on the technique used for repair, the tissue produced may include fibrous tissue, degenerative hyalinelike tissue, fibrocartilage, and bone (8). The type of tissue created will determine the long-term clinical success or failure. Factors affecting the quality of the repair are the age of the patient, the lesion size, the depth of the lesion, any associated ligament instability or
meniscus loss, any angular malalignment, and the acuteness of the injury when treatment occurs.
meniscus loss, any angular malalignment, and the acuteness of the injury when treatment occurs.
History
Patients presenting with chondral damage report various mechanisms of injury. These mechanisms often include a pivoting or twisting fall, a significant direct impact on the knee, an ACL tear, or a patellar dislocation. The differential diagnosis of any patient presenting with a traumatic hemarthrosis also should include a traumatic chondral lesion. Sometimes, however, the patient remembers no clearly traumatic event and only reports pain with weight bearing.
Patients commonly report the onset of pain localized to one particular compartment or a persistent, dull, aching pain that worsens after activity and may be most noticeable when the patient tries to fall asleep. Loaded activities, such as running, stair climbing, rising from a chair, and squatting, aggravate these symptoms. Sitting for prolonged times, such as in an automobile, a theater, or an airplane, sometimes aggravates pain from a patellar lesion.
In addition to pain, the patient may complain of swelling, crepitus, and giving way, catching, and locking of the knee. These tend to be induced by activity but vary widely from patient to patient.
Physical Examination
Joint line tenderness or an effusion sometimes is present depending on the patient’s activity before the evaluation. The alignment of the limb is important to evaluate; this includes looking for varus or valgus alignment, hyperextensile joints, or a flexion contracture.
For patellar or trochlear lesions, subpatellar crepitus, patellar grind, and pull-through sensitivity often are observed. Patellar maltracking, the Q angle, and tightness of the lateral retinaculum should be evaluated. Associated lesions should be considered, along with meniscal signs and joint instability.
Imaging
In the office, a standard radiographic evaluation should be performed. This includes a standing anteroposterior view of both legs in full extension to look for angular changes and to compare height of the joint space. If this view is not revealing, then a 45-degree flexion, posteroanterior, weight-bearing view to identify subtle joint space narrowing should be obtained. A non–weight-bearing lateral view with 45-degree flexion in which the posterior femoral condyles overlap, an axial view of both patellae to evaluate the patellar alignment, and an anteroposterior, knee-flexion view to outline the femoral intercondylar notch also should be obtained.
Magnetic resonance imaging can help to outline the surface of the articular cartilage and demonstrate localized, full-thickness lesions in a patient with otherwise normal standard radiographs. A layer of fluid or edema surrounding an articular cartilage lesion suggests that it is detached. The two most widely used imaging techniques are the T1-weighted, fat-suppressed, three-dimensional spoiled gradient echo technique and the T2-weighted, fast spin-echo technique. Software advances and newer MR imaging techniques with intravenous or intra-articular enhancement are continuing to improve the capability of MR imaging for the evaluation of articular cartilage.
Classification
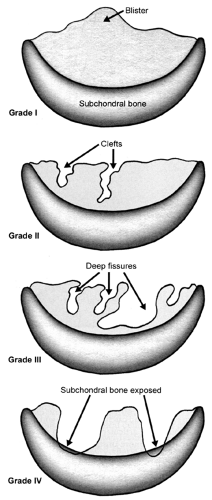
Evaluating articular cartilage lesions is important to facilitate communication, to arrive at a prognosis, and to devise an appropriate treatment plan. An evaluation should consider not only the size and depth of the lesion but also its location, any damage to the subchondral bone, and any associated pathology in the knee, such as an ACL or meniscal tear. The most common classification used for articular cartilage lesions is that of Outerbridge (9). In this system, articular cartilage damage of grade 1 shows softening or blistering of the surface; grade 2 shows fibrillation or superficial fissures of less than 1 cm in diameter; grade 3 shows deeps fissuring extending to the subchondral bone, without exposed bone, and measuring more than 1 cm in diameter; and grade 4 shows exposed subchondral bone (Fig 43.1-2). More recently, the Modified International Cartilage Repair Society Chondral Injury Classification System (10), or ICRS, has been developed. This system is based on the depth and amount of the cartilage injury. Using this system, ICRS grade 1 injuries are superficial, with a soft indentation or superficial fissures and cracks; ICRS grade 2 lesions involve less than half the cartilage depth; ICRS grade 3 lesions involve half or more of the cartilage depth, but not into the subchondral bone; and ICRS grade 4 lesions are osteochondral in extent.
Athletic injuries commonly injure the meniscal cartilage and, sometimes, the ACL as well. Injury to these structures can have a profound effect on the outcome of treatment of articular cartilage lesions. The meniscus cartilage functions to distribute loads more evenly across the joint surface, to improve cartilage nutrition, and to absorb shock. Loss of some or all of a meniscus can lead to osteoarthritis. This relationship makes preservation of the meniscus whenever possible a component of the treatment strategy to reduce articular cartilage injury. Continued ligamentous instability in the knee leads to additional articular and meniscal damage. When instability coexists with articular cartilage damage, the knee should be stabilized at the same time as the articular cartilage is repaired; otherwise, additional damage will compromise any articular cartilage procedure.
Any significant angular change in the leg (valgus or varus alignment) also should be corrected in conjunction with
articular cartilage surgery. If the malalignment is not corrected, then the articular cartilage will be subjected to increased pressures, and the result of the surgery will be compromised.
articular cartilage surgery. If the malalignment is not corrected, then the articular cartilage will be subjected to increased pressures, and the result of the surgery will be compromised.
Decision-making Algorithms
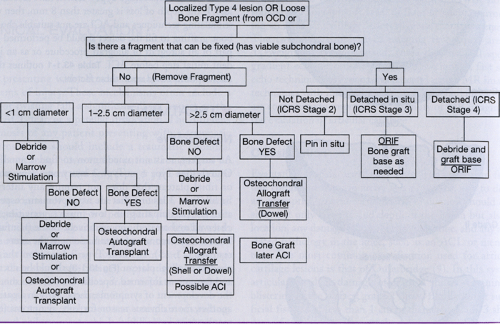
Treatment algorithms depend on many factors, but size and depth of the associated lesions are paramount. In addition, if a mobile fragment is associated with the lesion, then the possibility for reattachment exists only if the subchondral bone is viable. Some Outerbridge Type 4 lesions have associated bone loss. If the depth of loss is greater than 8 mm, then marrow-stimulating techniques and ACI are not suitable choices, and bone grafting to fill the defect should be performed either as part of an autograft or allograft procedure or as an independent initial step before ACI. Table 43.1-1 outlines the treatment options based upon these factors.
Nonoperative
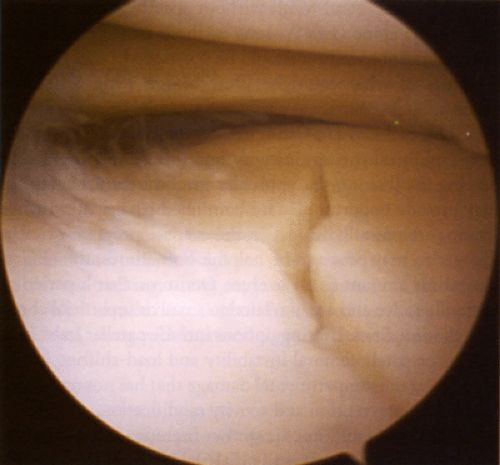
At surgery, it is not uncommon to find a small area of Outerbridge Type 1 or Type 2 injury on a femoral condyle or tibial plateau that does not require any intervention. Small Type 3 lesions that are asymptomatic, especially in athletes participating in low-impact activities, can be observed and treated nonoperatively. Some painful Type 3 lesions may become asymptomatic once the acute synovitis resolves (11,12,13). A linear Type 3 lesion on the femoral condyle or tibial plateau (Fig 43.1-3) should be documented and the patient informed. Specific intervention should await the development of symptoms, particularly in a patient with another, more obvious reason for knee symptoms (e.g., torn meniscus or loose body). Long-term studies suggest that isolated chondral lesions of less than 1 cm in diameter have an excellent or good result without specific treatment and may be left alone (14). As long as these areas do not cause symptoms, intervention should be postponed.
Areas of what could be Outerbridge Type 3 damage observed at MR imaging also warrant a trial of nonoperative care. This includes a course of nonsteroidal anti-inflammatory drugs, physical therapy, activity modification, and possibly, bracing. Evaluating the particular position the athlete plays may provide opportunities for continued participation while “resting” the joint. Changes in the exercise program or technique also may prove to be helpful. Sometimes, the clinical symptoms amount to an overuse syndrome that a period of rest will resolve and are unrelated to small or superficial chondral abnormalities. Bracing options include patellar stabilizing braces for patellofemoral instability and load-shifting braces for angular unicompartmental damage that has not responded to surgical intervention and activity modification.
Other available medications include intra-articular injections of hyaluronic acid (15,16). On occasion, an intra-articular injection of steroid can be helpful. At this point, no conclusive evidence indicates that nutritional supplements are beneficial for the anagement of partial- or full-thickness chondral lesions.
Nonoperative treatment for an athlete often is chosen because of the particular sport’s season. Operating on a player in the middle of his or her season can be a difficult choice, and surgery often is delayed until the season is over. This may be less than ideal. The future impact of this choice
may be profound to the athlete, however, and the decision should be made only after a candid and comprehensive discussion of the injury, its prognosis, and the potential consequences of the various treatment options by the physician with the athlete and, when appropriate, the athlete’s family.
may be profound to the athlete, however, and the decision should be made only after a candid and comprehensive discussion of the injury, its prognosis, and the potential consequences of the various treatment options by the physician with the athlete and, when appropriate, the athlete’s family.
Operative Indications
The indications for surgical intervention include symptomatic Outerbridge Type 3 lesions that have failed an appropriate nonoperative trial, Type 4 areas, and damage associated with loose fragments. The goal of this surgery is to relieve the symptoms of pain, swelling, and catching, locking, and giving way as well as to stabilize the articular cartilage to prevent further deterioration and to remove unstable, loose fragments. Some lesions, such as unstable osteochondritis dissecans fragments with viable subchondral bone or fresh traumatic fragments with viable bone, can be treated by primary repair (i.e., refixation to the bone bed). Larger articular cartilage lesions (diameter, >2 cm), especially those with loose fragments, deserve immediate intervention, because the chances of successful nonoperative treatment are low but, if this condition is allowed to go untreated, the risks of more damage are high.
Debridement
Debridement of Outerbridge Type 3 and Type 4 lesions as a primary treatment is appropriate for smaller lesions, low-demand patients, and incidentally observed lesions with few symptoms (Fig 43.1-4). The best results seem to be in younger patients with symptoms of less than 1 year in duration, a specific history of trauma, no previous surgery, little to no malalignment, and a low body mass index (17). Debridement
should only remove unstable chondral fragments that may cause mechanical symptoms and that are likely to become detached with additional activity or trauma. One study reported good to excellent results in 23 consecutive soccer players treated for partial- and full-thickness chondral flaps by arthroscopic debridement with a 1-year follow-up (18). Mechanical debridement does not stimulate articular cartilage repair (19), and there is a risk that the adjacent hyaline cartilage can be damaged (19,20). With additional trauma, these other areas can proceed to osteoarthritis (21).
should only remove unstable chondral fragments that may cause mechanical symptoms and that are likely to become detached with additional activity or trauma. One study reported good to excellent results in 23 consecutive soccer players treated for partial- and full-thickness chondral flaps by arthroscopic debridement with a 1-year follow-up (18). Mechanical debridement does not stimulate articular cartilage repair (19), and there is a risk that the adjacent hyaline cartilage can be damaged (19,20). With additional trauma, these other areas can proceed to osteoarthritis (21).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree