CHAPTER 69 Cervicogenic Headache
INTRODUCTION
It is a common experience in clinical practice to encounter syndromes that are diagnosed and treated using a variety of methods despite limited research and/or a lack of evidence-based medical consensus. Cervicogenic headache (CH) is one of these entities. Although there is long-standing notion that headaches can originate from structures in the neck and can be treated by interventions directed at the cervical spine, it is only during the past two decades that the topic has gained attention in mainstream medical literature. CH is a syndrome and not a distinct disease process. Its symptoms constitute a ‘final common pathway’ for pain emanating from a variety of cervical biomechanical and/or inflammatory disorders. It is a diagnosis of exclusion and more serious pathology should be ruled out. Specific spinal structures such as nerves, nerve root ganglia, uncovertebral joints, intervertebral discs, facet joints, ligaments, and even muscle may give rise to similar symptoms ultimately diagnosed as CH.1,2 The absence of pathognomonic symptoms, physical findings, or imaging studies makes the diagnosis and treatment of CH challenging to the clinician. This chapter will review the history, epidemiology, pathophysiological basis, diagnostic criteria, and treatment of CH.
HISTORICAL PERSPECTIVE
One of the earliest references describing a relationship between headache and the cervical spine was a series of lectures by Hilton occurring between 1860 and 1862, which were reported by Pearce.3 Almost 90 years later, a case series published by Hunter and Mayfield4 in 1949 formed the intellectual rationale for interventional techniques to treat cervicogenic headaches. Hunter and Mayfield reported that occipital neuralgia could be an important cause of headaches, with pain radiating from the occiput to the periorbital and jaw areas. Their theory was used to justify the injection of analgesics around the occipital nerves in an attempt to relieve these headaches. That same year, Bartschi-Rochaix5 coined the term ‘cervical migraine’ to label headaches presumed to derive from the neck. In 1955, Kovacs6 postulated that motion restriction of the cervical spine could lead to muscle spasm, thereby compromising the vertebral artery and nerves, and manifesting as a headache. This premise helped popularize osteopathic, chiropractic, and manual treatment of the cervical spine to relieve CH. Bogduk and Marsland7 in 1986 put forth their theory of ‘third occipital headache’ and advocated surgical intervention to treat it. This paper provided the first compelling scientific evidence that headaches could indeed develop as a consequence of a cervical spine biomechanical disorder.
The term ‘cervicogenic headache’ was initially introduced into the medical lexicon in 1983 by Sjaastad et al.,8 to describe patients with a headache associated with disorders of the neck. In 1988, the International Headache Society (IHS)9 amended its diagnostic classification system to include a category for CH. In 1990, Sjaastad et al.1 published specific diagnostic criteria for CH. Less stringent diagnostic criteria for CH was subsequently published by the International Association for the Study of Pain (IASP) in 1994,10 and by the Quebec Headache Study Group in 1995.11 In 1998, Sjaastad et al.12 revised their diagnostic criteria for CH based on more extensive clinical research.
EPIDEMIOLOGY
Few epidemiologic studies exist and these have only been done in the last decade.13–20 These studies support that CH is common. However, there is a great deal of variation in the perceived prevalence of CH. In the general population, for example, prevalence rates ranged from 0.4% to 2.5%,13,14 whereas studies looking at all patients with a complaint of headache reported estimates of 15–20%.9,14–16 The highest variation was among headache center patients, with prevalence estimates of 0.4–80%.17,18 The wide variation in reported prevalence can be attributed to the different diagnostic criteria used to define CH. The population pool each of the publications drew from was not comparable. Another factor influencing prevalence rates in headache centers is the overlap between the diagnosis of CH, tension-type headache (TTH), and common migraine headache (MH). Bono et al.19 report that 75% of patients fulfilling IHS criteria for MH also met most of the criteria for CH. One study of headache center patients reported that whereas only 16.1% were diagnosed with CH, an additional 20.1% were diagnosed with both MH and CH, for a total prevalence of 36.2%.16 Another study reported that 56.4% of CH diagnoses occur in combination with other headaches, including MH, TTH, and drug-induced headache.20
Analysis of patient descriptive data from studies where such information was given reveals that there is a preponderance of female patients with CH,21–23 with an average gender distribution of 79.1% female and 20.9% male. The mean age was noted to be 42.9 years, and the mean duration of symptoms was 6.8 years.24
NEUROANATOMIC BASIS AND PATHOPHYSIOLOGIC MECHANISMS
Convergence theory
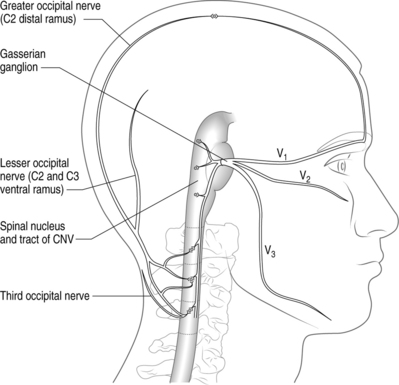
There is compelling circumstantial evidence that substantiates the theory of convergence to explain how cervical spine pathology can manifest as CH. The basic premise of convergence is that when primary afferents from two separate regions in the body converge on the same second-order neurons in the spinal cord, nociceptive activity along one of the afferent nerves can be perceived as pain in the other afferent nerve.25 Anatomically, both cervical and cranial afferent nerves innervate the head and face. The greater occipital, lesser occipital, and greater auricular nerves may innervate as far as the coronal sutures. Pain perceived in the forehead could be due to convergence between the trigeminal and upper cervical afferents. The cervicotrigeminal interneuron relay conveys nociceptive information to the upper cervical cord neurons. The trigeminal nucleus begins in the pyramidal decussations and descends as far caudad as C4 as the nucleus caudalis. The trigeminal nucleus is morphologically and functionally associated with the upper cervical cord and the cells form a column which is continuous with the posterior horn of the cervical cord. These anatomical relationships clearly demonstrate convergence between the trigeminal and cervical cord (Fig. 69.1). This convergence may also help to explain the systemic and sympathetic nervous system features accompanying CH.
Kerr26 discussed the relationship of the descending tract of the trigeminal nerve to the upper cervical roots following his dissection and analyses of adult cats. He observed that trigeminal afferents formed a bundle (trigeminosolitary bundle) with the solitary nucleus leading him to conclude that the trigeminal nerve provides a visceral component to the head and neck. The descending afferent trigeminal tract was identified as far caudal as the third cervical root. Trigeminal fibers were found at the low medullary, first, and third cervical cord levels. The second cervical root of the trigeminal fibers descends to the upper half of the cervical segment, but rapidly disappeared.26 Sectioned dorsal rhizotomy specimens demonstrated that the first cervical root traversed the trigeminal tract. There was no evidence of afferent fibers descending to the fourth through sixth cervical roots.26 The spinal tract of the trigeminal nerve entered the cervical cord in Lissauer’s tract as far down as the uppermost area of the third cervical cord. These cadaveric findings provide the anatomic basis for convergence theory (Kerr principle) and may explain why cervicogenic pain can occur ipsilaterally or bilaterally.
Almost every structure in the cervical spine has been implicated as a cause of headaches. Similarly, the mechanism of action may be due to degenerative changes, a direct result of trauma or without any underlying biomechanical basis. Current theories of anatomic causes of CH are based on retrospective observation, of either a reproducible finding on clinical examination, a response to stimulation of the structure, or relief of symptoms after treatment directed at the structure. Examples include the response of patients to surgery for disc disease,27 injections of posterior facets with local anesthetics,28 and injections of cervical muscles with botulinum toxin.29
Cervical facet joint
The zygapophyseal joints are implicated as a major source for cervicogenic headaches. The medial branch of the dorsal ramus above and below its location innervates the facet joints, except C2–3. The C2–3 is innervated by the superficial medial branch of the C3 dorsal ramus, also known as the third occipital nerve (TON).25,30 Pain patterns from stimulation of the cervical zygapophyseal joints have been studied in normal volunteers31 and from clinical evaluation.32,33 These studies suggest that the cervical zygapophyseal joints produce characteristic pain patterns according to the segmental distribution.31,32 The prevalence of zygapophyseal joint pain after whiplash has been reported as high as 50% in the C2–3 joint and 49% in the lower cervical joints.34,35
Cervical discs
Cervical discs have been implicated as a possible source of cervicogenic headache.25,36 Provocative discography is required to diagnose a painful cervical disc. In provocative discography, approximately 1 cc of radiopaque contrast is injected into the nucleus pulposus and the patient’s pain response is documented.37 Grubb and Kelly38 reported the prevalence of cervical pathology and referral patterns over a 12-year period. There were characteristic pain patterns depending on the level of the intervertebral disc. The C2–3 disc level referred pain into the upper cervical area, often extending into the occipital region and head, possibly accompanied by headaches in the occiput or frontal region. The pain pattern at the C3–4 level was similar to the C2–3 pain, but extended less into the occiput; and fewer patients experienced headache. More than 50% of the patients had three levels that had concordant responses, which may change clinical decisions to operate. Schellhas et al.,39 while correlating MRI findings with discography results, found a significant number of annular tears on discography that magnetic resonance imaging (MRI) was unable to detect. They concluded that magnetic resonance imaging is not reliable to delineate annular tears and should be used only as screening tool. Slipman et al.40 performed symptom mapping on 41 patients who underwent provocative discography at 101 levels to outline the pain referral patterns. The pain pattern on provocation of the C2–3, C3–4, C4–5, and C5–6 disc levels involved the occipital and/or facial areas, implicating the cervical discs as a possible source of CH.
Cervical segmental nerves
Diagnostic blocks of segmental nerves C2 and C3 have been routinely performed to confirm the diagnosis of cervicogenic headache.41 Bovin et al.28 performed an anesthetic blockade of the C2–5 spinal nerves to determine their involvement in the pathogenesis of cervicogenic headache. They reported that the most convincing relief occurred with a blockade of the C2 nerve. No patients responded completely to isolated blockades of nerves C3, C4, or C5. The C2 and C3 pain dermatomes were well described by Poletti in 1991.42
Occipital nerves
Another commonly implicated structure is the occipital nerve. The greater occipital nerve originates from the dorsal ramus of the C2 spinal nerve, the lesser occipital nerve from the ventral ramus of C2 and C3 spinal nerves via the cervical plexus, and the third (least) occipital nerve is the superficial medial branch of the C3 dorsal ramus (Fig. 69.2).43 Attributing occipital pain to irritation of the greater and lesser occipital nerve was common in the past. There is no compelling evidence that occipital pain is the result of irritation of the greater or lesser occipital nerve. Lancinating occipital neuralgia is recorded as a feature of temporal arteritis,44 in which case inflammation of the occipital artery could affect the companion nerve. In the majority of cases of so-called ‘occipital neuralgia,’ however, no such pathology is evident. The commonly held but inaccurate view is that occipital neuralgia is caused by entrapment of the greater occipital nerve where it pierces the trapezius. Surgical studies do not provide any evidence of this.45–47 The medial branch of C2 dorsal ramus, known conventionally as the greater occipital nerve, at first runs transversely across obliquus inferior. Near the origin of the obliquus inferior, the greater occipital nerve turns upwards across the dorsal surface of rectus capitis posterior major (see Fig. 69.2). Here it receives a communicating branch from the third occipital nerve. It emerges onto the scalp, not by piercing the trapezius, but by passing above an aponeurotic sling. This sling blends medially and laterally, with the aponeurotic insertions of trapezius and sternocleidomastoid, respectively, and thereby attaches to the superior nuchal line. Along its middle portion, the sling lies suspended from the superior nuchal line, leaving an aperture between it and the bone, through which the greater occipital nerve and the occipital artery emerge, leaving the plane deep to trapezius and sternocleidomastoid to enter the scalp.47 When the trapezius and sternocleidomastoid muscles contract, there is a ‘sling effect’ that actually relieves pressure on the greater occipital nerve.
The cardinal diagnostic criterion for greater occipital neuralgia seems to be response to blocks of the greater occipital nerve; but these blocks are not target specific when they involve volumes such as 5 mL45 or 10 mL.48,49 In such volumes, they do not selectively implicate the greater occipital nerve.
Bogduk and Marsland first explained the concept of the third occipital nerve (TON) headache.7 They provided a detailed description of the anatomy of the C3 dorsal ramus. The superficial medial branch of the C3 ramus (also known as the third occipital nerve) crosses the lateral and dorsal aspects of the lower half of the C2–3 zygapophyseal joint (see Fig. 69.2). It then passes across the lamina of C3 before turning backwards and upwards to pierce semispinalis capitis and splenius capitis to become cutaneous over the suboccipital area.7 In a later study, 100 consecutive patients with neck pain for more than 3 months were examined to determine the prevalence of TON headache.50 Seventy-one patients complained of headache associated with neck pain; in 40 of these patients, headache was the dominant complaint. In 31 patients, headache was the ‘secondary’ complaint. The prevalence of TON headache was 27%. Of those patients with headache as the dominant complaint, the prevalence was 53%.50
Dural attachments
Another theory of CH etiology comes from anatomical studies showing an attachment of the suboccipital tissues to the dura mater at the cervical–cranial junction, and the observation that mechanical traction on these tissues can cause movement of the dura.51–53 The rectus capitus posterior minor muscle53 and ligamentum nuchae52 have been shown to have direct connections to the suboccipital dura on very delicate dissection in a small number of cadavers. This suggests the possibility of the dura as a nociceptive structure in CH.
Inflammation
Recent studies have implicated inflammation as the cause of various spine-related conditions, including CH. When intervertebral discs are injured they have been found to release inflammatory mediators.54–56 Interleukin (IL)-1β and TNF-α increase the molecular events of inflammation.57 As well, a marked increase in the nitric oxide (NO) pathway has been demonstrated in patients with migraines or cluster headaches.58 Martelletti et al. observed increased levels of IL-1β and TNF-α in patients experiencing cervicogenic headaches during periods of spontaneous fluctuating basal pain and during mechanically induced attacks. There were statistically significant differences in cytokine levels as compared to controls.59,60 An increase in NO formation in the presence of reactive oxygen species may interact with IL-1β and TNF-α. This signals a cascade of proinflammatory/pain mediators such as prostaglandin and bradykinin, which play a role in neuronal sensitization. These interactions and responses implicate CH as an inflammatory consequence of cervical trauma. More importantly, they suggest that a myriad of pathological processes in different structures can manifest with similar or identical symptomatology (CH).
The inability to find a singular involved anatomic structure or pathology as the cause of CH has led some to believe that CH does not represent a single pathological entity, but rather a pain syndrome resulting from the nociceptive stimulation of almost any structure in the cervical spine.61
Differential diagnosis
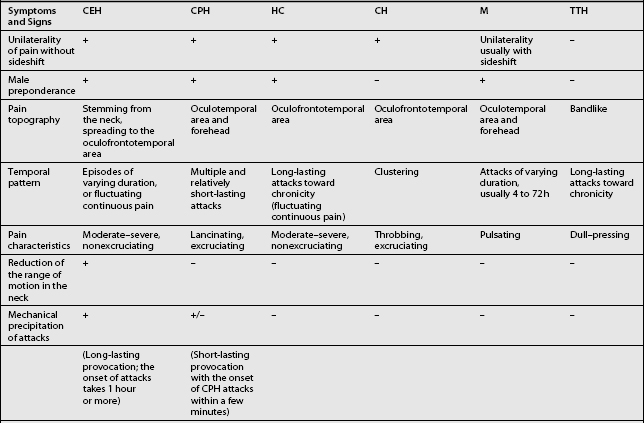
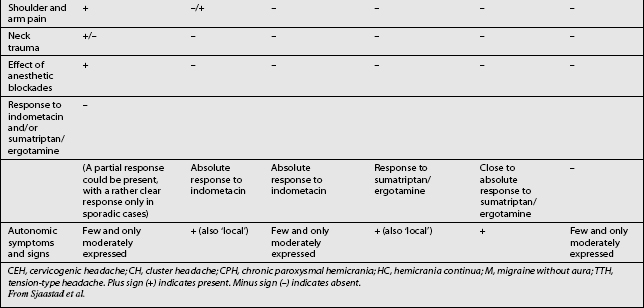
Other headaches, such as cluster headache, migraine (MH), chronic paroxysmal hemicrania (CPH), hemicrania continua (HC), and tension-type headache (TTH) must be included in the differential diagnosis (Table 69.1). Intracranial pathology, infection, neoplasm must be ruled out prior to assigning the patient the diagnosis of CH. Headaches associated with sinusitis, temporomandibular joint syndrome, and visual or auditory disturbances are rarely confused with CH because each possesses unique distinguishing characteristics.
CLINICAL PRESENTATION AND DIAGNOSTIC CRITERIA
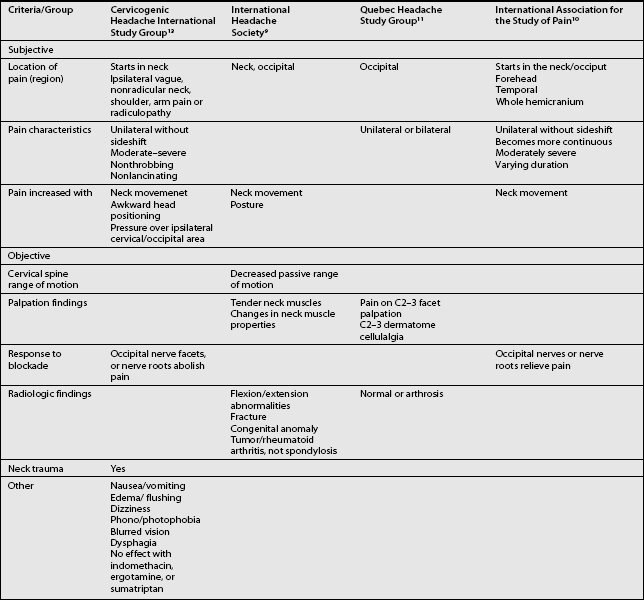
The term CH, although adopted by a number of organizations, is not universally accepted. Given this lack of consensus it is not surprising that a variety of labels are used to discuss headaches associated with disorders of the cervical spine. Perusal of literature published prior to 1983 emphasizes this point. Before that date, a number of terms such as vertebragenous headache,62 vertebrogenic headache,63 spondylotic headache,64 cervical spine syndrome,65 cervical migraine,66 cervical headache,67 cervicogenic syndrome,68 greater occipital neuralgia,69 and third occipital headache8 appear to have referred to the same clinical entity. Providing a consistent label for CH is not the only aspect of this entity that has been afflicted by a lack of unanimity. A similar problem arises when one considers the diagnostic criteria for CH. The most widely used diagnostic criteria are those proposed by Sjaastad in 1990, which were subsequently updated in 1998.12 These criteria have been adapted by the Cervicogenic Headache International Study Group (Table 69.2). Three other expert groups, the International Headache Society (Table 69.3),9 the Quebec Headache Group,11 and the International Association for the Study of Pain10 have published their own criteria. Table 69.4 summarizes the prominent features of the diagnostic criteria published by various expert groups.
Table 69.2 The Cervicogenic Headache International Study Group
From Sjaastad et al.13
Table 69.3 International Headache Society Criteria for Headache Associated with Disorder of the Neck
| Resistance to or limitation of passive neck movements |
| Changes in neck muscle contour, texture, tone, or response to active and passive stretching and contraction |
| Abnormal tenderness of neck muscles |
| Movement abnormalities in flexion/extension |
| Abnormal posture |
| Fractures, congenital abnormalities, bone tumors, rheumatoid arthritis, or other distinct pathology (not spondylosis or osteochondrosis) |
Comment: Cervical headaches are associated with movement abnormalities in cervical intervertebral segments. The disorder may be located in the joints or ligaments. The abnormal movement may occur in any component of intervertebral movement, and is manifest during either active or passive examination of the movement.
Adapted from IHS, Headache Classification Committee of the International Headache Society. Cephalalgia 1988.9
Obtaining an accurate history is the initial step in formulating a differential diagnosis. A history of neck/head trauma should be considered to be of importance, especially if there is a possible whiplash mechanism.2,12 It has been reported that whiplash injuries usually affect the cervical facet joint, intervertebral disc, cervical nerve root, or a combination of these structures.34,70–72 Prior to the modification of the diagnostic criteria by Sjaastad et al. in 1998,12 CH had been defined as a unilateral headache spreading to the neck and the ipsilateral shoulder/arm, triggered by head/neck movements and posture. A strict unilaterality requirement has been softened in the updated CH diagnostic criteria.12 Since CH is a syndrome, the pathologic process can involve the contralateral side, potentially presenting as a bilateral headache. Even in the typical unilateral case, pain may eventually spread to the opposite side particular when the headache becomes severe. Nevertheless, the symptom intensity will remain greater on the original side.12
Other diagnostic features of CH include signs and symptoms of neck involvement. Such signs are biomechanical precipitation of attacks, whether iatrogenically and subjectively induced, reduced active range of motion (ROM) in the neck in one or more directions, diffuse ipsilateral neck/shoulder/arm pain of nonradicular nature, and occasionally, seemingly radicular arm pain (see Table 69.2). Pain may be reproduced iatrogenically by external pressure (1) over the tendinous insertions in the occipital area, (2) along the course of the major occipital nerve, (3) over the groove immediately behind the mastoid process, (4) over the upper part of the sternocleidomastoid muscle on the symptomatic side, and (5) over the lateral aspect of a cervical zygapophyseal joint. Pain may be precipitated intrinsically by neck movements and/or sustained, awkward head positioning, especially during sleep.
The duration of pain attacks, a few hours to a few weeks, and pain intensity, vary widely in CH with a strong tendency toward chronicity. CH can present as episodic in the initial phase becoming chronic in later stages. The pain usually starts in the neck, eventually spreading to the oculofrontotemporal area. Symptoms may actually be more intense in this latter location than in the occipital or cervical region.1,12 The duration of pain episodes for CH is frequently longer than in common migraine; the pain intensity is moderate and nonexcruciating, unlike cluster headache, and it is usually of a nonthrobbing nature. Autonomic symptoms and signs, such as photophobia, phonophobia, nausea, vomiting, and ipsilateral periocular edema, are generally less frequent and less marked than in common migraine, but they can occur.1,2,12,21,22,73 Swallowing difficulty is reported, albeit rarely occurring associated phenomenon.74 There have also been cases with features consistent with a CH picture, but with additional dizziness/vertigo and vertebral drop-attacks.8,73
In the revised criteria, among the ‘Other Important Characteristics’ (see Table 69.2), the lack of complete response to indomethacin, sumatriptan, and ergotamine has also been introduced.
Authors’ recommendation
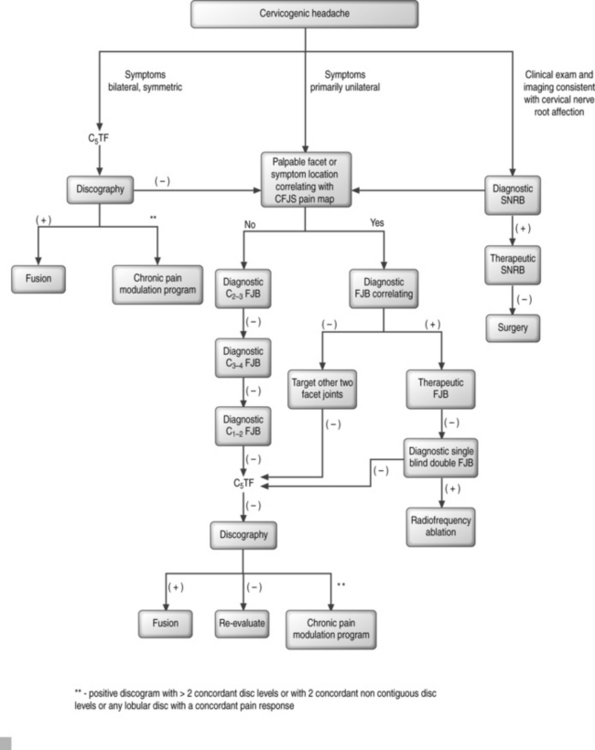
Formulating a probability analysis of the structures involved is the most important step in the diagnostic and therapeutic algorithm (Fig. 69.3). Unilateral symptoms of occipital headaches greater than neck pain following a traumatic event is more suggestive of cervical facet joint syndrome (CFJS) than internal disc disruption.34,70 Similarly, one may suspect CFJS more than internal disc disruption if there is focal tenderness following palpation of an isolated cervical joint or if the patient is able to point to the painful area corresponding to the distribution of pain reported for a particular facet joint.75 Examination finding of increased focal suboccipital pain with terminal cervical flexion and sequential lateral rotation suggests pain emanating from a C1–2 joint. Bilateral symptoms of neck pain with headaches would be more suggestive of cervical internal disc disruption (CIDD) syndrome. Reproduction of symptoms by provocative maneuvers, which facilitate closure or narrowing of the neuroforamina, are positive, then nerve root involvement rather than facet joint syndrome or internal disc disruption syndrome is of higher probability. In whiplash injuries, CFJS may be more common than upper cervical nerve root injury;34,76 however, this may be a consequence of the paucity of epidemiological data for whiplash-induced cervical nerve root injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree