CHAPTER 5 Central Influences on Pain
NEUROPLASTICITY AND THE DISEASE OF PAIN
Phenomenologically, the disease of pain represents a concatenation of biological, psychological, and social events that disrupt the life of the patient. Cascades that occur in each area result in the experience of chronic pain. When people experience pain, there is an expectation that it will eventually stop as the body heals. If this fails to occur there are consequences that occur throughout the individual’s life. Mersky and Bogduk define pain in the following manner: ‘Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.’1 The reason that this definition stands the test of time is that it succinctly reflects the ambiguity of pain and unifies the physical and the emotional, actual injury and potential damage, expectations and experience, while highlighting the unpleasantness of pain. What it fails to take into account is the distinction of the symptom of acute pain and the disease of chronic pain. Donald Price describes acute pain as a two-staged event of desire to avoid harm and expectation that harm will be avoided. Price points out that when a person is injured, immediate concern about tissue damage is replaced with anxiety about healing and ‘threat to the self.’2 He describes fear and anger as emotional aspects of acute pain and despair, frustration, hopelessness and depression as common affective experiences of persistent pain. While this expands the understanding of pain from the IASP definition it still lacks the necessary social perspective. For this we must turn to the biopsychosocial model developed by George Engel. Engel’s view of the biomedical model was that it ignored anything beyond reductionistic science and did not consider patients in the context of their psychology or as a larger part of society. In his paper, ‘Clinical Application of the Biopsychosocial Model,’ Engel stated, ‘The biomedical model can make provision for neither the person as a whole nor for data of a psychological or social nature … The triumphs of the biomedical model all have been in the areas for which the model has provided a suitable framework for scientific study. The biopsychosocial model extends that framework to heretofore neglected areas.’
Neuroplasticity develops in response to injury. This is usually a temporary state aimed at reestablishing the homeostatic balance of both the PNS and CNS. Peripherally, the immune system responds to injured tissue with release of inflammatory chemicals, such as bradykinins, prostaglandins, and histamines to isolate the injury from healthy tissue and increase the number, availability, and activation of peripheral opioid receptors.4 When this works well, healing occurs and the CNS and PNS quickly remodel to normal molecular, physiologic, and anatomic status. When there is unrelenting peripheral stimulation or a breakdown in adaptive function, neuroplastic changes that occur at the peripheral injury, dorsal horn of the spinal cord, and the brain result in the disease of pain, also known as maldynia.5 This is very different from the symptom of pain, eudynia. In maldynia pathological processes developing at the site of the peripheral injury, and discussed in much greater detail in Chapters 3 and 4, include excessive activity of sodium and calcium channels, ectopy through the alteration of neurotransmission from orderly ion channel transductions to rapid-fire stimulus encoding, and recruitment of non-nociceptors proximal and distal to the site of injury.6 At the dorsal horn of the spinal cord, excessive activity of excitatory amino acids results in wind-up pain, driven by the extreme peripheral and central input. Wind-up appears to be the direct product of activation of N-methyl D-aspartate (NMDA) receptors in the superficial dorsal horn neurons.7 After nerve injury, reorganization directed by the dorsal root ganglion (DRG) results in dorsal horn atrophy of nociceptive (Aδ and C-fibers) end-terminals, as well as phenotypic changes in non-nociceptors (Aβ fibers) so that they produce nociceptive neurotransmitters and grow and reconnect into areas that are normally populated by nociceptive (C-fiber) nerve terminals.8 The brain itself goes through major changes at both a molecular and anatomic level. Actual apoptosis occurs in the brain in different regions for different chronic pain states, resulting in nerve cell loss and dendritic pruning.9 Functional MRI and MR spectroscopy in complex regional pain syndrome (CRPS) and in chronic low back pain have demonstrated this phenomenon.10
ACUTE PAIN
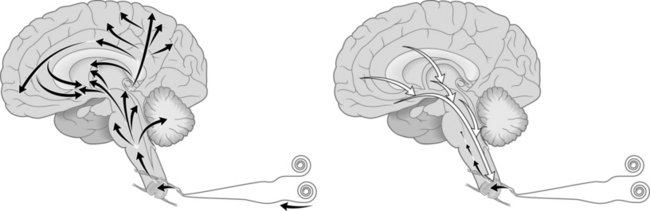
When a peripheral nociceptor is activated an action potential is generated and sent down the axon to cell bodies in the dorsal root ganglion. The incoming signal results in the DNA encoding the messenger RNA to instruct ribosomes in the cytoplasm to manufacture substance-P (SP), a neurotransmitter and calcitonin gene related peptide (CGRP), a neuropeptide. SP is constantly being manufactured at the ribosome in response to pain signals. It is then sent to nerve terminals where it is released. It is important to remember that the pain signal’s effect upon the nerve cell body causes the manufacture of more SP. SP is released at nerve endings synapsing on second-order nociresponsive neurons within the spinal cord. There it activates neurokinin-1 receptors (NK-1R) in the spinal cord dorsal horn cells and a new action potential is generated.11 In acute pain the signal at these dorsal horn neurons is typically equivalent in strength to the signal coming into the CNS. This signal is then passed predominantly to either the neospinothalamic tract or the paleospinothalamic tract and sent to midbrain, thalamic, hypothalamic, and limbic structures in the brain. These are passed further to other deep and cortical structures in multiple regions of the brain resulting in a broad stimulation of pain sensation, arousal, autonomic pathways, somatosensory centers, and neuroendocrine pathways. This allows for a response to a painful stimulus, which is sensory and emotional and produces physiologic activity that is orchestrated through the interplay of far-flung areas of the brain and culminates in a countersignal from other widespread regions of the brain via descending pathways to dampen and ultimately to help to extinguish the incoming signals (Fig 5.1).12
CHRONIC PAIN
Chronic pain is best thought of as persistent pain that has no significant chance of being altered by usual treatment modalities or natural healing. This is pain that often has been amplified by the severity of pain signals reaching the CNS and causing a wind-up phenomenon at the dorsal horn of the spinal cord.13 It may even involve rapid depolarization and ectopy at the site of injury or the cell bodies in the dorsal root ganglia. Sympathetic coupling, antidromic neurogenic inflammation, CNS immune cell mediated inflammation, limbic kindling, activation of the neuromatrix, or other forms of central sensitization may also be involved.9 Finally, anxiety, depression, or other psychosocial factors are influenced by and influence pain perception.14 The result is pain that is physiologically amplified and often psychologically augmented, while conversely activating psychological responses. The patient experiences pain that feels overwhelming and represents an unwelcome change in life.
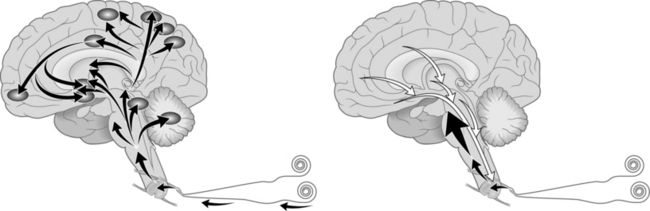
When a peripheral nociceptor is chronically activated, SP, CGRP, and adenosine triphosphate (ATP), which targets the P2X3 subtype purinergic receptor, are released from the nociceptor and in turn activate non-nociceptive touch, movement, position, temperature and mechanical receptors. Action potentials are generated and sent down the axon to their cell bodies in the dorsal root ganglion. The incoming signal results in the DNA encoding the messenger RNA to instruct ribosomes in the cytoplasm to make SP in cells that normally do not produce this neuropeptide. In particular, SP is released at the dendritic end-terminals into the synapses between the dendritic tree and the dorsal horn interneurons. On the interneuron membranes SP activates NK-1R in the spinal cord dorsal horn cells and new action potentials are generated. At the dorsal horn the SP/NK-1R complex is incorporated into the cytoplasm of the neuron, where it is ultimately broken down into its constituent components and recirculated. The action of the SP/NK-1R complex also depolarizes the membrane wall of the neuron and causes the removal of magnesium ions from NMDA receptors. Magnesium ions normally block these receptors, keeping them inactive. This results in activation of normally dormant NMDA receptors via reception of the ubiquitous excitatory neurotransmitter, glutamate, which in turn allows calcium ions to pass into the interneuron cytoplasm, causing the signal to be amplified in frequency and duration (wind-up).15 This amplified signal is then passed through Lissauer’s tracts to the neospinothalamic tract and/or the paleospinothalamic tract and sent to brain stem, midbrain, thalamic, hypothalamic, and limbic structures in the brain. To complicate matters further, other pathways engaged in chronic pain by unknown, but documented, mechanisms augment these classical spinal cord tracts.16 These signals are passed further to other deep and cortical structures in multiple regions of the brain resulting in a broad stimulation of pain sensation, arousal, autonomic responses, somatosensory activation, and neuroendocrine alteration. This results in an extreme response to a painful stimulus, which is sensory and emotional and produces physiologic activity that is modulated by sensory input from multiple areas of the brain.17 The brain’s attempt to diminish this signal is thwarted due to the ongoing process of wind-up pain at the dorsal horn of the spinal cord (Fig 5.2).
CENTRAL PAIN
Trauma to specific areas of the central nervous system can result in severe pain that may be immediate or delayed for months to years after the damage occurs. The term often used for this is central pain, and although many central pain processes drive this type of pain, the name actually describes the location of pain in the CNS. Central pain can be a problem in a number of different pathological CNS processes. Strokes in areas of the brain that involve ascending or descending pain pathways or the thalamus itself are frequently marked by severe and unrelenting pain. Demyelinating illnesses may cause central pain. Trauma to the spinal cord can also result in chronic pain. This type of pain is subject to the same problems noted in centralization of peripheral pain, including neurotransmitter, neurocircuit, and neuroreceptor plasticity. Central pain of this type can be rather difficult to treat due to limited utility of a number of medications. Different studies show that after injury to the spinal cord, anywhere from 18% to 94% (with an average of 69%) of patients will develop chronic pain disorders and that chronic pain ranks high on the list for patients with difficult post-traumatic sequelae of spinal cord injury, right behind paralysis, sexual dysfunction, and incontinence. Recent studies show that about one-third of patients who develop postspinal cord injury pain will suffer from severe pain.18 Syringomyelia is an example of central pain that develops slowly over time due to deafferentation, a physiologic interruption of input to CNS neurons resulting in loss of excitation, inhibition, and modulation of information to the receptive neurons. In syringomyelia more than 90% of patients will develop pain disorders, but the time of onset averages 4–9 years after development of the syrinx.19 This delay in symptoms can obfuscate the etiology of the pain and has led to the incorrect conclusion by some researchers that syringomyelia is rarely painful. Studies done over time on patients with syrinx show that development of chronic pain is a common phenomenon.
Melzak’s latest theory views deafferentation pain as a problem of hypervigilant brain function, via a concept he labels the neuromatrix. Deafferentation occurs when the normal input to central nociceptors is interrupted. This brain-based matrix consists of neurons, circuits, and dendrites in the thalamus, cortex, and limbic system, constantly cycling signals, allowing the brain to generate body image with or without sensory input. Interruption of normal peripheral input forces these centers of the brain to provide information to other areas of the brain. When consistent input to the matrix is interrupted, it works to reestablish balance by approximating output consistent with that previous input. Melzak further theorizes that a second output neuromatrix sends information to the PNS and its innervations, resulting in perception of movement, spasm, and pain even if these sites are denervated (spinal root avulsion) or amputated. At the same time, the output neuromatrix sends signals to the emotional centers of the brain, evoking emotional response to pain. The neuromatrix preserves the image of self at the cost of unrelenting pain. Thus, the failure of brain-based homeostatic mechanisms leads to central pain.20
WIND-UP PAIN
Normally, NMDA receptors are located on all CNS neurons, but are in an inactive state due to a magnesium block. They serve the purpose of destroying dying nerve cells by apoptosis to promote removal of cells and pruning back of dendrites to make room for cell replacement with new cells and rearborization of the dendritic tree. Apoptosis is best understood as programmed cell death and consists of complex intracellular process that lead to activation of NMDA receptors on the cell membrane and influx of calcium into the cytosome, resulting in pinpoint neuronal death, dendritic pruning, and ultimate replacement with new neurons, arborization, and synaptogenesis. It is this way that the CNS makes new connections, new pathways, and reduces dead and redundant cells and circuits. Without this process, the vibrant interplay of anatomic, physiologic, emotional, and experiential events could neither be sustained nor adaptively altered.21
To understand wind-up pain it is essential to understand the role of neurocircuits at the junction of the PNS to the dorsal horn, tracts from the spinal cord to the brain, intracranial connections, and pathways from the brain back to the dorsal horn. Furthermore, the role of neurotransmitters and neuromodulators must also be appreciated, as the combination of ubiquitous compounds with more focal substances results in the picture of adaptive processes gone wrong. Ultimately, intracellular processes that result in exertion of genomic shifts in the proteome determine when and where neurotransmitters, neuroreceptors, neuropeptides, and neurohormones will be dispatched.
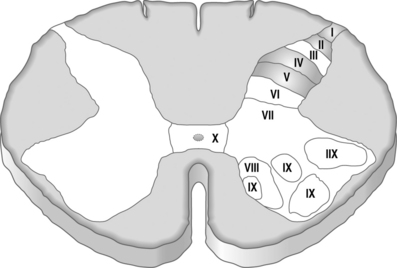
The dorsal horn is organized in lamina with second-order neurons serving as receptors for incoming signals from PNS nerve terminals. There are ten lamina identified as part of the organization of the spinal cord with lamina I–VI as part of the dorsal horn.22 Lamina I, II, IV, and V are the main nociceptive regions of the spinal cord.11 Fast excitatory postsynaptic potentials are produced by excitatory amino acid neurotransmitters and slow excitatory postsynaptic potentials are produced by neuropeptides (Fig 5.3).23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree