Matthew Bartels
![]()
11: Cardiopulmonary Rehabilitation
![]()
The goal of achieving competency in cardiopulmonary rehabilitation is to be able to provide rehabilitation for two groups of patients. There are patients with primary cardiac and pulmonary disease who need cardiac/pulmonary rehabilitation and then there are patients with other disabilities who have a cardiac or pulmonary secondary disability. This includes patients with respiratory failure and patients who have need for ventilatory support. The incidence of dual-disability patients is now rising as more rehabilitation patients are elderly and have multiple comorbidities. Applying the principles of cardiac rehabilitation to the programs of these patients broadens the number of patients with stroke, vascular disease, or other conditions who can be included in active cardiac and pulmonary rehabilitation programs.
In order to apply cardiac and pulmonary rehabilitation for patients with cardiopulmonary disease, it is necessary to understand the basic principles of cardiac and pulmonary physiology and apply these principles to improve the exercise capacity of these patients. The principles of normal exercise physiology also need to be understood in order to apply the correct adaptations for patients with abnormal cardiopulmonary physiology.
A basic understanding of the historical and present models of cardiopulmonary rehabilitation along with an assessment of several systems in which these are currently delivered will be reviewed and will include inpatient and outpatient settings as well as the need for developing methods of allowing for maintenance programs and potential use of telemedicine.
Cardiac and pulmonary rehabilitation are among the most underutilized yet most effective treatments for patients with cardiopulmonary disease. It is essential for rehabilitation specialists to know how to provide cardiopulmonary rehabilitation and to have an important role in the delivery of cardiopulmonary rehabilitation services to patients with primary and secondary cardiopulmonary disability.
PATIENT CARE
GOALS
Provide competent patient care that is compassionate, appropriate, and effective for the evaluation, treatment, education, and advocacy for patients diagnosed with cardiopulmonary disorders all along the health care continuum.
OBJECTIVES
1. Describe the key components of the assessment of the patient with cardiac and pulmonary diseases.
2. Formulate comprehensive interdisciplinary rehabilitation treatment plans for the patient with cardiac and pulmonary diseases.
3. Evaluate individuals diagnosed with cardiac and pulmonary diseases for physical impairments, activity limitations, and participation restrictions.
ASSESSMENT OF CARDIOPULMONARY FUNCTION
History and Physical Examination
It is essential to have a complete cardiopulmonary history and physical examination as part of the evaluation for patients with cardiac or pulmonary disease who are to undergo rehabilitation. The history may reveal issues to be addressed and help to develop the rehabilitation program. Important parts of the history are from both verbal and nonverbal cues and will allow the patient and physician to establish mutual goals and improve compliance with the treatment program.
History
Historical information should include the patient’s emotional state, concurrent illnesses, other disabilities, functional history, occupational history, social history, personal habits, family dynamics, and the effect of disability and cardiopulmonary illness on the patient’s performance in the community. It is essential that symptoms at rest and with activity are reviewed. Some specific aspects of the history are discussed in the following text (1).
Dyspnea. Shortness of breath (SOB) is often the chief presenting complaint for patients with cardiopulmonary disease. The description of dyspnea needs to be complete in order to differentiate the contribution of cardiac or pulmonary disease to the underlying dyspnea. Pulmonary causes of dyspnea include pulmonary vascular disease, restrictive lung disease, and obstructive lung disease. Primary cardiac issues include ischemic heart disease, congestive heart failure (CHF), valvular heart disease, and arrhythmias. Often, both cardiac and pulmonary problems may be present simultaneously. In both conditions, physical conditioning should be ascertained. Patients should also have assessment of their psychological state as they may also have anxiety, which heightens dyspnea. Dyspnea may or may not be associated with hypoxemia and this should be assessed as well with pulse oximetry. See Table 11.1 for an outline of common causes of dyspnea. Chest Pain. Chest pain, tightness, and burning are the classic symptoms of coronary insufficiency, but they may also be present in patients with valvular heart disease or arrhythmia. The history may help differentiate the causes of chest pain and the duration, quality, provocation, and location of the pain; in addition, any ameliorating factors should be noted. Precipitating factors for chest pain are of particular interest as they may affect the design of the therapy program and may be a cause of functional limitations experienced by the patient. There may also be chest pain associated with certain lung conditions, including pressure and tension with both obstructive and restrictive lung disease. Chest pressure is also commonly experienced with exertion in patients with pulmonary vascular disease.
TABLE 11.1 Causes of Dyspnea
DISORDER | SITE OF PATHOLOGY | PATHOPHYSIOLOGY |
Airflow limitation | Lung | Mechanical limitation to ventilation |
Restriction (intrinsic) | Lung | Poor lung compliance |
Restriction (extrinsic) | Chest wall | Poor chest wall compliance |
Valvular disease | Heart | Limited cardiac output |
Coronary disease | Heart | Coronary insufficiency |
Heart failure | Heart | Limited cardiac output |
Anemia | Blood | Limited oxygen-carrying capacity |
Peripheral circulation | Peripheral vessels | Inadequate oxygen supply to metabolically active tissues |
Obesity | Adipose tissue | Increased work of movement Respiratory restriction if severe |
Psychogenic | Emotional | Hyperventilation |
Deconditioning | Multiple organ systems | Loss of ability to effectively distribute systemic blood flow |
Malingering | Emotional | Inconsistent results |
Acute pulmonary disease | Lungs | Increased V/Q mismatch |
Palpitation. Palpitations are a sensation of an irregular or forceful heartbeat and can be indicative of serious arrhythmias.
Syncope. Cardiac syncope is usually abrupt with no warning or only a brief warning (with the patient feeling as if he or she were about to pass out). Pulmonary syncope is often slower in onset and may be due to hypercarbia, hypoxemia, or pulmonary vascular disease. Syncope may indicate the presence of aortic stenosis, idiopathic hypertrophic subaortic stenosis (IHSS), primary pulmonary hypertension (PH), hypercarbia, hypoxemia, ventricular arrhythmias, reentrant arrhythmias, high-degree atrioventricular (AV) block, or sick sinus syndrome. Postural syncope can be due to autonomic dysfunction, neurological disease, vagal stimuli, or psychological stimuli.
Edema. Peripheral edema may be an indication of CHF, and in pulmonary disease may indicate right heart failure and PH.
Fatigue. Fatigue is common in cardiopulmonary disease, and there may be other causes that may coexist that contribute to fatigue. A good history can help to identify depression, physical exhaustion, medication side effects, and deconditioning.
Cough. Cough is common in restrictive and interstitial lung disease (ILD) and is often a component of obstructive lung disease, with or without sputum production. Cough may also be caused by cardiac congestion. “Cardiac” cough is characterized by postural changes, with little or no sputum production, and is often relieved by assuming an upright position. Cardiac cough is often nocturnal and episodic.
Limitations of the History. Often patients may not be able to give a complete or accurate history, and even though history does not allow for risk assessment or exercise prescription, it is still an essential part of cardiopulmonary rehabilitation evaluation.
PHYSICAL EXAMINATION
The physical examination of the cardiopulmonary patient is complex, and a full review is beyond the scope of this chapter. Still, we will review some of the important unique elements. The general survey of the patient can reveal exophthalmos (which might be a clue to thyrotoxicosis) or xanthelasma (indicating hypercholesterolemia). Extremities can reveal acrocyanosis (chronic hypoxemia) or clubbing (chronic hypoxemia). Ankylosis is associated with aortic valve disease and conduction defects, and Down syndrome may have associated cardiac abnormalities. Myasthenia or neuromuscular disease may be related to both cardiomyopathy or conduction disease and ventilatory failure.
The details of the cardiopulmonary examination are well described in basic physical examination textbooks, so only a few key points are highlighted here.
Cardiac auscultation can show a fixed splitting of the second heart sound, which can indicate an atrial septal defect. A murmur may indicate valvular heart disease. PH typically produces a heightened second heart sound, and a mid-systolic click may indicate mitral valve prolapse. A noncompliant ventricle can be detected via an atrial gallop at the cardiac apex, and a left ventricular gallop may reveal heart failure. Aortic valve sclerosis, which is common in older patients, may cause an aortic systolic murmur. The combination of pulse contour, the nature of the splitting of the heart sounds, and the quality of the murmur can help differentiate aortic sclerosis from aortic stenosis. Younger patients should be evaluated for pulmonary stenosis and valvular heart disease and may be hard to differentiate from IHSS. Diastolic murmurs can hint at mitral stenosis or PH with pulmonary valve regurgitation. Continuous murmurs may indicate ventricular septal or atrial septal defect. The physical examination can then lead to further evaluation, which may then avoid complications in cardiac rehabilitation.
Pulmonary examination may reveal decreased breath sounds (obstructive disease) or a barrel chest with increased anteroposterior (AP) diameter. Patients may have diffuse crackles or basilar crackles that indicate ILD. There may be inspiratory stridor indicating upper airway obstruction or expiratory wheezing or rhonchi that indicate obstruction or secretions. Patients with suspected respiratory compromise should also have assessment of their symmetry of breathing, looking for accessory muscle use and for possible compromise to diaphragmatic function.
Cardiopulmonary examination and history are important in the detection of patients at risk for complications in a cardiac rehabilitation program, which can often be started in the physiatrist’s office with the basic history and physical examination.
The rehabilitation of the patient with cardiac disorders commonly includes the following: (a) education about risk factor modification, lifestyle modification, stress management, and medications; and (b) conditioning and strengthening programs. The rehabilitation of the patient with a pulmonary disease includes the following: (a) strengthening and conditioning exercise programs; (b) smoking cessation; and (c) education about nutrition, stress and anxiety management, medications, supplemental oxygen use, and breathing techniques.
Most of the activity limitation and participation restriction that arises from cardiopulmonary disease stems from the exercise limitation that goes along with impaired cardiopulmonary function. Fatigue is a very common issue, with most patients with heart failure and chronic obstructive pulmonary disease (COPD) reporting chronic fatigue. A major symptom limiting activity in both cardiac and pulmonary patients is dyspnea, with chest pain more common in cardiac patients. Hypoxemia is the major physical limitation, leading to activity limitation and participation restriction in pulmonary patients, while decreased cardiac output (CO) is the most common physical limitation, leading to activity limitation and participation restriction in patients with cardiac diseases. These limitations (fatigue, hypoxemia, dyspnea, chest pain, and low CO) can be deceptive to detect and many observers will not appreciate the degree of disability that they can cause, in their worst forms limiting patients to their homes or bedrooms and causing major limitations in their lives.
MEDICAL KNOWLEDGE
GOALS
Demonstrate knowledge of established evidence-based and evolving biomedical, clinical, epidemiological, and sociobehavioral sciences pertaining to cardiac and pulmonary diseases, as well as the application of this knowledge to lessen impact of physical and functional impairments and guide treatment.
OBJECTIVES
1. Review important principles of cardiac and pulmonary anatomy, as well as normal and abnormal cardiac and pulmonary physiology.
2. Review general principles of cardiac and pulmonary rehabilitation.
3. Review principles of cardiac rehabilitation following myocardial infarction (MI), revascularization procedures, post-cardiac transplantation, and in patients with cardiomyopathy, valve disease and cardiac arrhythmias.
4. Review principles of pulmonary rehabilitation in diseases such as emphysema, intrinsic lung disease, ventilatory failure, and PH.
5. Review principles of cardiac and pulmonary rehabilitation as they apply to physically disabled populations.
REVIEW OF CORONARY ANATOMY AND PHYSIOLOGY
Cardiac Anatomy
All physicians involved in cardiac rehabilitation need to be familiar with the normal distribution of the major arteries of the heart, cardiac valvular anatomy, and the structures at risk from ischemia or infarction in these distributions.
Overall, the heart consists of paired atria and ventricles, with deoxygenated venous blood entering the right atrium, traversing the right ventricle via the tricuspid valve, entering the pulmonary artery through the pulmonic valve. Oxygenated blood reenters the heart via the left atrium, entering the left ventricle through the mitral valve, where it is then ejected into the aorta through the aortic valve. Proper heart valve function ensures unidirectional unobstructed flow of blood, and proper atrial function can help to augment cardiac function, adding up to 15% to 20% to the total CO. Atrial contribution is greater with increased heart rate (HR) and in conditions with decreased ventricular compliance (2). The loss of the contribution of atrial “kick” is especially important to consider in disease conditions where atrial dysfunction is seen, such as atrial fibrillation.
The cardiac conduction system is a specialized system of muscle cells (myocytes), which allow coordinated contraction of the atria and ventricles at a controlled rate. The SA node is the usual cardiac pacemaker located in the right atrium. The electrical pulse travels through three atrial internodal pathways to the AV node where conduction is delayed to cause sequential atrial and ventricular contraction. Below the AV node, the signal passes into the bundle of His and divides into left and right bundles. The left bundle has anterior and posterior fascicles, and all bundles then end in terminal branches to excite the myocytes and cause contraction. MI, aging, and other conditions can alter the conduction system and create heart block and sick sinus syndrome. Congenital defects and accessory pathways can be seen in Wolff-Parkinson-White (WPW) syndrome.
Variation of Arteries
Normally, there can be several different variations of coronary circulation. The left main coronary artery usually divides into the left anterior descending and the circumflex arteries, while the right coronary artery continues on as a single vessel. The most common anatomy (60%) is right dominant circulation, while left dominant circulation (10%–15%) is seen when the posterior descending artery arises from the left circumflex. The remaining 30% of individuals have balanced circulation where the posterior descending arises from the left circumflex and right coronary arteries (2) (Table 11.2).
Cardiac Physiology
Cardiac myocytes are highly metabolically active with nearly 65% oxygen extraction at all levels of activity (compared with 36% for the brain and 26% for the rest of the body). Carbohydrates are the preferred energy source (40%), with fatty acids making up most of the remaining 60%. This high oxygen extraction and coronary blood flow only during diastole predisposes the heart to ischemic injury, especially in the endocardium. Normally, coronary vasodilation increases blood flow with exertion, via nitric oxide pathways. It is the goal of most medical and surgical therapies for ischemia to restore or preserve myocardial perfusion, through vasodilation or bypass or endovascular procedures. Exercise is also a very effective therapy, as regular exercise can increase cardiac collateral circulation and improve arteriolar vasodilation (2).
Appropriate fluid balance is also important in cardiac care, as adequate venous return can maintain appropriate cardiac “preload,” while fluid overload with excessive venous return can exacerbate heart failure. For mechanical cardiac constriction, surgery can allow greater dilation of the ventricle to restore CO, and in dilated heart failure, medical treatment may decrease the size of the ventricles in order to increase CO. Left ventricular assist devices (LVADs) and cardiac transplantation can be used for the most refractory cases.
REVIEW OF PULMONARY ANATOMY AND PHYSIOLOGY
Pulmonary Anatomy
Basic pulmonary anatomy includes the upper and lower airways (the oropharynx, larynx, trachea, mainstem bronchi, and smaller bronchi), the lung parenchyma itself, and the chest walls and musculature (diaphragm, accessory muscles of breathing, rib cage, pleura). Abnormalities in any of these structures can cause pulmonary limitations and may lead to decreased exercise capacity. The pulmonary vasculature includes pulmonary arteries and veins, which deliver deoxygenated blood to the lungs and deliver oxygenated blood to the left atrium, as well as intrinsic pulmonary artery circulation, which delivers oxygenated blood to the respiratory tree.
TABLE 11.2 Coronary Artery Anatomy
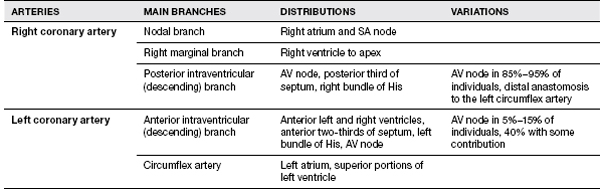
Upper airway obstruction from vocal cord paralysis or tumor may cause stridor. Reactive airway disease may cause asthma and be associated with dyspnea. Patients with parenchymal lung disease may have a loss of alveoli with loss of intrinsic recoil of the lung and subsequent hyperinflation and dyspnea (emphysema, COPD) or may have interstitial scarring with increased recoil and decreased ability to diffuse oxygen through the lung tissues (ILD, pulmonary fibrosis). In some conditions there may be an interplay of both restrictive and obstructive diseases, although usually one is predominant over another (cystic fibrosis, sarcoidosis). Assessment of the lung parenchyma can be made with imaging or physiological testing such as pulmonary function tests (1).
Pulmonary Physiology
Normal breathing is governed by the respiratory center in the medulla oblongata of the brain. Injury to that part of the brain can cause respiratory failure and need for ventilatory support. Signals for inspiration are carried via the phrenic and other somatic nerves to the diaphragm and secondary inspiratory muscles (intercostals, sternocleidomastoids, pectorals) and cause rhythmic breathing via negative pressure in the chest wall. Normal exhalation is a passive process dependent on elastic recoil of the chest wall and the lung parenchyma. In pulmonary disease such as COPD and emphysema, exhalation can become active with the need for abdominal muscles to cause exhalation and markedly increasing the work of breathing. Any disease affecting the brain, spine, or phrenic nerves; the muscles; or the mechanical properties of the chest wall or diaphragm will affect normal respiration. In ILD, the compliance of the lung tissue can become so severe that the lung volumes decrease and hypoventilation can result (1).
Pulmonary vascular disease can lead to PH, and this can be either primary or secondary. Primary pulmonary vascular disease can be idiopathic or can result from vasculitis, thromboembolic disease, or as a part of the progression of intrinsic parenchymal disease. Secondary hypertension is usually a result of left heart failure and may eventually develop a component of intrinsic vascular compromise. Chronic exposure to hypoxemia may also create pulmonary vascular compromise and can be seen with obesity, obstructive sleep apnea, and high-altitude exposure. Hypoxemia leads to pulmonary vascular constriction and in a chronic state will lead to vascular intimal hypertrophy and the development of fixed pulmonary vascular resistance and PH.
Basic Terminology
AEROBIC CAPACITY Aerobic capacity (VO2max) is the measure of the work capacity of an individual. Aerobic capacity is expressed as the oxygen consumed by the individual (liters of oxygen per minute or in milliliters of oxygen per kilogram per minute). Oxygen consumption (VO2) increases linearly with workload, increasing up to a plateau, which occurs at the VO2max. Knowing a person’s maximal exercise capacity can help with assessing disability and planning exercise and recovery programs (1).
HEART RATE HR is used to guide exercise as it has a linear relationship to VO2. Maximum HR is determined by age and can be estimated as: peak HR = 220 – age or by the Karvonen equation. The slope of the relationship of HR and VO2 is determined by physical conditioning, with a lower slope representing improved conditioning. HR is mediated by the interaction of vagal and sympathetic tone and circulating catecholamines and can be altered by medications (1).
STROKE VOLUME Stroke volume (SV) is the quantity of blood ejected with each left ventricular contraction. Normally, maximal SV can be increased with exercise. SV increases the most during early exercise and is sensitive to postural changes, changing little when one is supine but increasing in a curvilinear fashion until it reaches maximum at approximately 40% of VO2max. SV declines with advancing age, in cardiac conditions that result in decreased compliance, after MI, and in heart failure (1).
CARDIAC OUTPUT This is the product of the HR and SV and increases linearly with work. Peak CO is the primary determinant of VO2 max and is maximized in upright work compared with supine work (1).
MYOCARDIAL OXYGEN CONSUMPTION Myocardial oxygen consumption (MVO2) is the oxygen consumption of the heart and rises in a linear fashion with workload. Angina occurs when the MVO2 exceeds the maximum coronary artery oxygen delivery. MVO2 can be estimated with the rate pressure product (RPP), calculated as the product of the HR and the systolic blood pressure (SBP) divided by 100. Upper extremity and isometric exercises have a higher MVO2 for a given VO2. Supine exercises demonstrate a higher MVO2 at low intensity and a lower MVO2 at high intensity compared with erect exercises. MVO2 is also increased in the cold, extreme heat, after smoking, or after eating (1).
For pulmonary exercise assessment, basic static lung volumes and dynamic responses to exercise need to be assessed. A full discussion of pulmonary function testing is beyond the scope of this chapter, but important values include the following:
Total Lung Capacity (TLC). The volume of air in the lungs at full inspiration.
Vital Capacity (VC). Volume change of air through the mouth between full inspiration and full expiration.
Forced Expired Vital Capacity (FVC). Maximum volume that can be expired from the lungs after a maximal forced expiration.
Forced Expiratory Volume in 1 Second (FEV1). The FEV over 1 second is the most commonly reported value and is severely limited in airway obstruction.
Maximal Voluntary Ventilation (MVV). Measurement of the maximum minute ventilation over 15 seconds.
Residual Volume (RV). Volume of air in the lungs after a full expiration.
Tidal Volume (TV). The volume of a regular nonforced breath at rest.
Diffusion of the Lung for Carbon Monoxide (DLCO). This measures the ability of gases to diffuse across the alveolar membrane.
The capacity to exercise in cardiac and pulmonary conditions is best assessed with cardiopulmonary exercise testing and is useful for diagnostic, prognostic, and exercise prescription in both populations. The effects of various physiological conditions on the interpretation of pulmonary exercise testing are shown in Table 11.3.
TABLE 11.3 Effects of Physiological Conditions on the Interpretation of Pulmonary Testing
ABNORMALITY | PHYSIOLOGIC ABNORMALITY | GAS EXCHANGE |
Obesity | Increased work with activity | Rapid alveolar–arterial pA–PaO2 fall with exercise |
Peripheral vascular disease | Claudication limits exercise | Low VO2max Low anaerobic threshold |
Pulmonary vascular disease | Impaired pulmonary blood flow | Decreased O2 uptake at maximum work Low anaerobic threshold Rapid pulse at low exercise |
Anemia | Low oxygen-carrying capacity | Low VO2max Low anaerobic threshold Rapid pulse at low exercise |
Chronic obstructive pulmonary disease | Restricted expiratory phase of breathing Decreased alveolar ventilation | Low VO2max Low anaerobic threshold Rapid pulse at low exercise Submaximal heart rate achieved |
Restrictive lung disease (intrinsic) | Poor diffusion capacity Poor pulmonary compliance | Low VO2max Low anaerobic threshold Tachypnea Low pulmonary reserve High alveolar–arterial pA–PaO2 difference |
Restrictive lung disease (extrinsic) | Poor pulmonary compliance | Low VO2max Low anaerobic threshold Tachypnea Low pulmonary reserve Submaximal heart rate achieved |
Asthma | Restricted expiratory phase of breathing Decreased alveolar ventilation In exercise-induced asthma, peak flows drop 5–10 min into exercise | Most findings normal when not symptomatic, resemble obstructive with acute attack |
Ventricular failure | Compromised pulmonary blood flow | Low VO2max Low anaerobic threshold Tachypnea Exaggerated heart rate response to exercise |
Ischemic heart disease | Chest pain/cardiac ischemia Can precipitate ventricular failure | Often normal Can appear like mild ventricular failure Can have inability to raise BP |
Metabolic acidosis | Metabolic acidosis, low HCO3 | Normal diffusion Exaggerated response of ventilation to exercise |
AEROBIC TRAINING
Aerobic training is a physical exercise that increases the cardiopulmonary capacity (VO2max). The basic prescription for aerobic training requires four components: intensity, duration, frequency, and specificity.
Intensity is how hard an exercise is and can be indicated by a target HR, metabolic level (MET level), or intensity (wattage). For cardiac training in primary prevention, the target HR can be set at 80% to 85% of the predicted maximum HR or peak HR on an exercise tolerance test (ETT). Exercises above 60% of the maximal HR will have training effect. This is also true in pulmonary disease.
Frequency is how often exercise is performed over a fixed time period, usually expressed in sessions per week. At a minimum, training programs should be done three times per week, increased to five times per week for low-intensity programs.
Specificity describes the activity to be done in exercise. Training benefits are most specific to the activities that are performed; for example, cycle ergometry is not as beneficial for improving walking as a treadmill training program. Specificity of a training program should take into account the needs of a particular patient. For example, upper arm ergometry for a spinal cord patient or cycle ergometry over treadmill for patients with lower limb arthritis. This is the law of specificity of conditioning and is commonly referred to in cardiopulmonary conditioning programs (1).
The benefits of aerobic training are as follows:
Aerobic Capacity. Increased with training. The resting VO2 does not change, and the VO2 at a given workload does not change. The changes are also specific to the muscle groups that are trained.
Cardiac Output. The maximum CO increases. Resting CO does not change, but resting HR will decrease, with increased SV. This leads to lower MVO2 at rest and submaximal exercise.
Heart Rate. The HR is lower at rest and at any given workload. Maximum HR is not changed.
Stroke Volume. SV is increased at rest and at all levels of exercise and allows for maintenance of CO at a given workload with a lower HR and RPP at a given level of exertion.
Myocardial Oxygen Capacity. The maximum MVO2 does not usually change but is lower at a given workload after training. This can allow individuals to perform more activities below the anginal threshold with fewer symptoms and increased safety. Pharmacological interventions or revascularization procedures can also improve maximum MVO2.
Peripheral Resistance. The peripheral vascular resistance (PR) decreases in response to exercise training. This is often referred to as “afterload.” The decrease in PR is due to the increased vasodilatation in peripheral vascular beds. This causes a lower RPP and a lower MVO2 at a given workload and at rest.
Minute Ventilation. As a person has improved conditioning, he or she will require a lower VO2, and thus a lower VE for a given activity. This can decrease dyspnea in patients who have pulmonary and cardiac diseases.
Tidal Volume. As patients improve with exercise, they can sustain a higher TV, meaning a decrease in respiratory rate and a decrease in subjective dyspnea.
Respiratory Rate. This will decrease as TV is able to improve and will be lower for a given minute ventilation, helping to lower dyspnea.
By remembering the basic physiology described earlier, cardiopulmonary rehabilitation specialists can improve function, decrease symptoms, and have a positive effect on outcomes in their patients. The main benefits of cardiac conditioning are reduced cardiac risk and improved cardiac conditioning. The reduction of cardiac risk is established historically in numerous studies. As long ago as 1989, pooled data from 22 randomized studies of exercise in 4,554 patients following acute MI demonstrated a 20% to 25% reduction in all-cause mortality, fatal MI, and cardiac mortality in a 3-year follow-up (3). The benefits of cardiac rehabilitation apply to the elderly, women, and in postbypass patients (3).
Pulmonary rehabilitation is also effective in COPD; it helps to decrease hospitalizations and improve function and quality of life (4–7). An overview of the pulmonary rehabilitation program is in Table 11.4.
TABLE 11.4 Goals and Methods of Pulmonary Rehabilitation
GOALS | METHODS |
Prevention |
|
Smoking cessation | Enroll in a cessation program, emotional support, monitor abstinence |
Immunization compliance | Assure proper immunizations, communicate with primary physician |
Prevent exacerbations | Self-assessment skills taught Self-intervention taught Instruct on accessing private physician |
Appropriate medication use | Review medications and dosing schedules Review interactions and side effects Review appropriate use of inhalers and nebulizers |
Pulmonary toilet | Review bronchial hygiene Teach proper cough techniques Use of chest physiotherapy as needed Teach chest physiotherapy techniques to family as appropriate |
Appropriate use of oxygen therapy | Teach use with exertion Review self-monitoring Review use of equipment Encourage acceptance of the need for O2 Review importance of use and consequences of failure to use oxygen |
Nutritional counseling | Counseling to achieve ideal body weight Counseling to avoid high carbohydrate diet Instruction in avoidance of high sodium diets Encourage balanced nutrition, avoidance of fad diets |
Teaching regarding: COPD Pulmonary toilet Medication use Oxygen use Family support group Counseling as needed | |
Dyspnea Relief—Exercise Training | |
Exercise | Multifaceted program individualized to each patient’s needs |
Strengthening | Emphasis on gradual increase in strength Focus on proximal muscle groups Avoid injury to weakened musculotendinous structures Focus more on high-repetition, low-intensity training |
Conditioning | Work to gradually increase exercise tolerance Cross-training program Emphasis on the development of an independent training program Increase ambulation endurance with gait training Appropriate oxygen titration during exercise |
Respiratory muscle training | Inspiratory and expiratory muscle training Isocapnic hyperpnea Inspiratory resistance training Inspiratory threshold training |
Upper extremity training | Increase strength Increase capacity for sustained work Improve shoulder girdle strength |
ADL training | Energy conservation techniques Adaptive techniques Relieve anxiety and stress Encourage pacing in activities |
Breathing retraining | Pursed lip breathing Diaphragmatic breathing |
Anxiety reduction | Stress relaxation techniques Paced breathing Autohypnosis Visualization Medications as needed Treat anxiety Treat depression |
Improve confidence | Build compensatory techniques Build confidence in ability to exercise |
Disease Management | |
Disease acceptance | Education regarding disease process Reassurance about aggressive treatment |
Coping skills | Support group Psychology and social work intervention as needed Treat depression as needed |
Quality of life improvement | Improve ADL tolerance Improve coping skills Improve disease management |
Advance directives review | Counseling regarding: Health care proxy Resuscitation orders Help in preparing paperwork |
Encouragement | Support group Social work support Psychological support |
Continuing compliance | Team encouragement Physician counseling Involve primary care physician in plan Family education |
Abnormal Physiology
Heart
Physiatrists should be familiar with the alterations of normal cardiac physiology in disease in order to order effective cardiac rehabilitation. Cardiac disease is due to either decreased CO or ischemic disease, with a degree of overlap. During ischemic episodes, the myocardium becomes less compliant with less contractility and a subsequent decrease in SV, while valvular heart disease decreases maximum CO through either stenotic valves (e.g., aortic or mitral stenosis) or valvular regurgitation (e.g., aortic or mitral insufficiency). Finally, CHF has decreased CO with low SV, associated with a lower VO2max, higher resting HRs, and often a greater MVO2 at a given VO2.
Arrhythmias usually decrease the CO through decreased SV and increased HRs. This may be due to a loss of atrial contribution (atrial “kick”) with supraventricular arrhythmias (e.g., atrial fibrillation of supraventricular tachycardias), or from high HRs without atrial coordination (e.g., ventricular tachycardias and ventricular bigeminy).
Surgical treatments of cardiac disease either aim to restore coronary circulation (e.g., bypass and intravascular procedures) or aim to restore normal anatomy (e.g., valve replacement). Medical therapy for ischemic disease aims to improve coronary circulation, and treatment for heart failure aims to decrease afterload, reduce fluid overload, and increase inotropy with surgical treatment including LVADs. Medical treatment of arrhythmias with medications has been difficult, but implantable defibrillators and pacemakers have been very efficacious treatment for these conditions. For intractable heart disease, cardiac transplantation or LVAD are final possible treatments. All of these conditions, including transplant and LVAD patients, benefit from cardiac rehabilitation. An understanding of the underlying physiology of all of these conditions is essential to successful rehabilitation. Pretransplant patients have abnormalities, including CHF, intractable ischemia, or arrhythmia, while posttransplant patients have persistently high resting HR and a limited ability to increase SV and peak exercise HRs that can limit exercise response. The outline for programs of rehabilitation of all of these syndromes is discussed subsequently (1).
Lung
Patients with pulmonary disease have essentially three types of impairment: obstructive or restrictive lung disease, or pulmonary vascular disease. Two of these may often be present in one patient and may increase the morbidity of the individual. A basic understanding of the underlying physiology will help in the design of the exercise program for the patients.
A basic way to think of pulmonary impairments is to divide intrinsic lung limitations into either obstructive or restrictive conditions. In obstructive lung disease, there is an inability to exhale, either due to upper airway or large airway disease (sleep apnea, tracheomalacia, vocal cord disease, asthma, bronchitis) or due to secretions or lung parenchymal disease (emphysema, bronchiectasis), and there may also be an acute condition (asthma) versus chronic condition (chronic obstructive lung disease [COPD]). The hallmark of COPD is carbon dioxide retention and active exhalation. Medical treatments in obstructive disease are aimed at relieving the obstruction via bronchodilation for reactive airways, use of steroids and antibiotics for inflamed or infected airways, or surgery for emphysema (lung volume reduction surgery [LVRS]). In end-stage disease, lung transplantation may be the only option.
For patients with restrictive lung disease, there is a limitation to tidal volumes from inability to expand the chest wall (extrinsic restriction) or from very noncompliant lung tissue (intrinsic restriction). Forms of extrinsic restriction include neuromuscular disease, paralysis, and kyphoscoliosis. These conditions often respond to mechanical ventilation and respiratory muscle training, if possible. The intrinsic restrictive lung diseases include pulmonary fibrosis and sarcoidosis and may have profound associated hypoxemia due to decreased diffusion capacity of scarred lung tissue. These patients classically have severe hypoxemia and may need high-flow supplemental oxygen. In the end stage of intrinsic restrictive disease, patients can suffer severe ventilatory failure with hypercarbia and hypoxemia. Since many of these conditions may be progressive, lung transplantation is often a treatment option in selected candidates with endstage disease. Table 11.5 shows some of the lung pathologies and effects on inspiratory reserve and RV (obstructive diseases), and Table 11.6 shows the effects of various conditions on lung compliance (restrictive diseases) (8).
TABLE 11.5 Lung Pathology
LOSS OF INSPIRATORY RESERVE | INCREASE IN THE RESIDUAL VOLUME |
Intrinsic | Intrinsic |
– Lung fibrosis | – Bronchial obstruction |
– Obliteration of alveoli | – Airways collapse |
– Pulmonary edema |
|
Extrinsic | Extrinsic |
– Chest wall rigidity | – Respiratory muscle weakness |
– Respiratory muscle weakness | – Chest wall rigidity |
– Chest wall restriction from bracing |
|
TABLE 11.6 Causes of Alterations in Lung Compliance
INCREASED COMPLIANCE | DECREASED COMPLIANCE |
Intrinsic | Intrinsic |
– Decreased elastic recoil | – Alveolar obliteration |
– Loss of alveolar walls | – Increased alveolar stiffness |
| – Increased alveolar wall thickness |
| – Decreased surfactant |
Extrinsic | Extrinsic |
– Flail chest | – Chest wall stiffness |
– Multiple rib fractures | – Chest wall deformity |
| – Chest wall bracing |
Cardiac Rehabilitation
Cardiac rehabilitation programs come in 2 forms: primary prevention, which includes risk factor modification and education before a cardiac event; and secondary prevention, which is cardiac rehabilitation after the establishment of cardiac disease and includes exercise and risk factor modification.
Primary prevention programs are not usually performed in a rehabilitation setting but are in primary care settings. Primary prevention includes a focus on the reduction of cardiac risk factors with education for at-risk patients and includes community-based cardiac disease prevention. Primary prevention can have a profound effect on the rate of cardiac disease with a decrease in obesity, blood pressure, and lipid profiles. Behavior modification should ideally begin in childhood in order to establish healthy behavior patterns to be maintained throughout life. This is also important in disabled populations who are generally sedentary and may have other risk factors. Primary prevention can include the use of medications to treat hypertension, lipid abnormalities, and antiplatelet agents. These are all cost-effective approaches and can decrease mortality and morbidity on a population-based scale, in addition to the individual benefits (9–11).
Secondary risk factor modification programs are an essential part of cardiac rehabilitation programs for individuals after onset of cardiac disease. Secondary risk factor modification programs include all of the features of primary prevention programs with the addition of disease-specific education and formal exercise. Smoking cessation is an essential part of both primary and secondary prevention programs (12–14).
Pulmonary Rehabilitation
Patients with pulmonary disease follow a similar pattern to cardiac patients with regard to their programs of rehabilitation. Since most pulmonary diseases are chronic and progressive, there is not a model of acute inpatient rehabilitation, but early mobilization programs are now working to limit debility in patients who undergo exacerbations. The essential components of outpatient rehabilitation for pulmonary disease are the same as for cardiac patients. Primary prevention for pulmonary disease includes smoking prevention and cessation, occupational safety, and prevention of exposure to environmental and infectious agents. Secondary prevention is in the form of medication adherence and education, smoking cessation, oxygen supplementation, and environmental modification to prevent recurrent exposure to environmental triggers (5,7).
For patients with ventilatory failure, lung transplant may be a treatment, and these patients can benefit from rehabilitation both before and after transplantation. Pretransplant rehabilitation is focused on the underlying condition and posttransplant rehabilitation is focused on education and restoration of muscle strength, which is impaired from the medical regimen for post-transplant patients.
Cardiac Rehabilitation of the Post-MI Patient
Standard cardiac rehabilitation following MI usually follows the classical model of cardiac rehabilitation, as first described by Wenger et al. in 1971 (1). Since revascularization is now common and infarcts are smaller than in the past, cardiac rehabilitation usually has three stages or phases, eliminating the classical stage 2 recovery phase. The comparison of the old and new programs of mobilization is seen in Table 11.6.
The exception to this paradigm is postsurgical patients who may need an intermediate recovery phase from their surgery before launching into the training phase of rehabilitation. To recount, the first phase is the acute phase in hospital period immediately following the cardiac event that ends at discharge. The second phase is the rehabilitation training phase, with intense education and aerobic conditioning to achieve the desired results of exercise. The third phase is the maintenance phase, which is devoted to the continued aerobic exercise and maintenance of lifestyle modifications. Risk factor modifications are taught and reemphasized throughout all phases. A similar model exists for pulmonary disease patients. All patients who are identified to have cardiopulmonary disease can start directly into the second phase without a hospitalization.
TABLE 11.7 Wenger Protocol—Then and Now
ORIGINAL PROGRAM | MODERN PROGRAM | ACTIVITY |
Day 1 | Day 1 | Passive range of motion (ROM), ankle pumps, introduction to the program, self-feeding |
Day 2 | Day 1 | As above, also dangle at side of bed |
Day 3 | Day 1 | Active assisted ROM, sitting upright in a chair, light recreation, and use of bedside commode |
Day 4 | Day 1 | Increased sitting time, light activities with minimal resistance, patient education |
Day 5 | Day 1 | Light activities with moderate resistance, unlimited sitting, seated ADL |
Day 6 | Day 2 | Increased resistance, walking to bathroom, standing ADL, up to 1-hr-long group meetings |
Day 7 | Day 2 | Walking up to 100 feet, standing warm-up exercises |
Day 8 | Day 2 | Increased walking, walk down stairs (not up), continued education |
Day 9 | Day 2 | Increased exercise program, review energy conservation and pacing techniques |
Day 10 | Day 3 | Increase exercises with light weights and ambulation, begin education on home exercise program |
Day 11 | Day 3 | Increased duration of activities |
Day 12 | Day 3 | Walk down two flights of stairs, continue to increase resistance in exercises |
Day 13 | Day 3 | Continue activities, education, and home exercise program teaching |
Day 14 | Day 3 | Walk up and down two flights of stairs, complete instruction in home exercise program and in energy conservation and pacing techniques |
Acute Phase (Phase 1)
The basics of the early mobilization program are outlined in Table 11.7. The educational program relating to risk factor modification should be introduced during phase 1 as many patients are ready to listen to advice in their acute hospitalization. With or without revascularization, the acute mobilization should be done with cardiac monitoring and under the supervision of a trained physical or occupational therapist or nurse. The post-MI HR rise with activity should be kept to within 20 beats per minute (bpm) of baseline and the SBP rise within 20 mmHg of baseline. Any decrease of SBP of 10 mmHg or more should be considered worrisome and exercise halted. The major goal of the phase 1 program is to condition the patient to perform activities up to four METs, which is within the range of most daily activities at home postdischarge.
For patients with pulmonary disease, similar goals exist with new emphasis now being placed on early mobilization in the intensive care unit (ICU), even while still on the ventilator. Treatments such as extracorporeal membrane oxygenation (ECMO) are also now coming to the fore and will allow for mobilization of patients since they will be alert and not sedated on a ventilator, thereby allowing the ability to walk with assistance in the ICU setting. After discharge, patients should be enrolled in pulmonary outpatient programs to allow for consolidation of early gains and a full program of education and exercise.
Inpatient Rehabilitation Phase (Phase 1B)
In order to distinguish between patients who have a rapid recovery after their cardiopulmonary event (pure phase 1) and those patients who require either acute or subacute rehabilitation treatment prior to discharge home, the designation of phase 1B rehabilitation has been established. With advanced age or significant comorbidities or other disabilities that make mobilization more difficult, many rehabilitation specialists will care for these phase 1B patients. The guidelines for exercise are the same as they are for the phase 1 patients, but with a longer recovery period extending their hospitalized care to an acute or subacute rehabilitation setting prior to discharge.
Training Phase (Phase 2)
The training phase of the cardiopulmonary rehabilitation is classically started after a symptom-limited full-level ETT for cardiac patients, or a Cardiopulmonary exercise test (CPET) for complex pulmonary patients. Target HR or intensities can be taken from the exercise test and used as guidelines during aerobic training. For low-risk patients, 85% of the maximum HR is generally regarded as safe. For individuals who are at greater risk, exercise programs at lower target HRs can be tailored to individual patients based on the results of the exercise test and the reason for cessation of exercise. Generally, for patients with life-threatening arrhythmias or chest pain, lower target HRs are chosen. Pulmonary patients with hypoxemia are given oxygen as needed up to high flow (15 L/min or more) in order to maintain saturation for safe exercise. In patients with higher risk, a target HR of 65% to 75% of maximum can be safe and effective in a regular exercise program (50), and target rates as low as 60% can still yield a training benefit. For the patients at higher risk, it is appropriate to monitor individuals at each increase in activity.
A classic cardiopulmonary training program is three sessions per week for approximately 8 to 12 weeks. The major limitation of cardiopulmonary rehabilitation is a lack of referral and a lack of facilities for rehabilitation in many areas. In order to assist in increasing access to cardiopulmonary rehabilitation, creative programs have been developed, including at-home programs for low-risk patients, telemedicine programs, and community- and home-based programs. A key to success in home-based programs is assuring that patients are able to perform self-monitoring during their exercise program. Guidelines for self-monitoring are outlined in the standard references (15,16). Just as in the supervised programs, all exercise sessions should begin with a stretching session, followed by a warm-up session, the training exercise, and end with a cool-down period. It is important to remember that conditioning benefit is related to the specificity of training, and that the conditioning applies to the specific muscles exercised.
Maintenance Phase (Phase 3)
Despite usually receiving the least attention, the maintenance phase of a cardiopulmonary rehabilitation is the most important part of the program. If the patient stops exercising, the benefits gained from phase 2 can be lost in a few weeks. From the beginning of the rehabilitation program, the importance of an ongoing exercise program needs to be emphasized and efforts need to be made to integrate exercise and lifestyle modifications into the patient’s life. For moderate-level exercises, patients should perform exercise at the target intensity learned in their rehabilitation program for at least 30 minutes 3 times a week. For low-level exercise, exercise should be done 5 times a week. For pulmonary patients, home pulse oximetry monitoring can be helpful and is cost-effective and easily learned (8).
Cardiac Rehabilitation Programs in Specific Conditions
Angina Pectoris
The goal of cardiac rehabilitation for angina is to decrease angina and improve fitness. The exercise benefit in angina is derived from improving efficiency and improved collateralization.
Cardiac Rehabilitation After Revascularization Procedures
Postcoronary Artery Bypass Grafting
Cardiac rehabilitation can consolidate the benefits after revascularization by emphasizing secondary prevention and adding the benefits of regular exercise to prevent recurrence of the coronary disease. Close monitoring needs to be considered for patients with low ejection fractions and CHF. Full programs of rehabilitation often need to be delayed for up to 6 weeks in patients with sternotomy to allow for sternal healing, but for patients without sternotomy, it can begin as soon as the patient has recovered from the procedure. The rehabilitation of patients after percutaneous interventions (PCIs) is essentially the same as after surgical bypass.
Cardiac Rehabilitation After Cardiac Transplant Surgery
Typically, cardiac transplant patients suffer from months of preoperative invalidism and general muscle weakness and have depression and anxiety. The transplant itself usually resolves the cardiac disability, but a comprehensive approach to the patient is necessary. Due to the complexity of the procedure and the occurrence of vascular and neurological complications, some of these patients also require phase 1B programs and may come to acute or subacute rehabilitation settings.
Because of cardiac deinnervation and immunosuppressive medications, the physiology of the posttransplant patient is altered from the normal cardiac patient. Transplantation causes cardiac denervation, with loss of both sympathetic and vagal connections to central regulation. Loss of vagal inhibition to the SA node creates a mild baseline tachycardia of 100 to 110 bpm. With exercise, there is no direct sympathetic stimulation to the heart and chronotopic response is mediated by circulating catecholamines, causing a blunted and delayed HR response to exercise. Peak HR is usually 20% to 25% lower than in matched controls. There is also resting hypertension, due to the renal effects of calcineurin inhibitors (e.g., cyclosporine and tacrolimus) and prednisone, and diastolic dysfunction may also be seen. Finally, maximum work output and maximum oxygen uptake are reduced to about two-thirds of the age-matched population, similar to lung transplant recipients. Transplant patients thus have higher than normal perceived exertion, minute ventilation, and ventilatory equivalent for oxygen at submaximal exercise levels. At maximum effort, transplant patients demonstrate lower work capacity, CO, HR, SBP, and oxygen uptake, while resting HR and SBP are higher than in normal individuals. Finally, both resting and exertional diastolic blood pressure are higher after cardiac transplantation than in normal individuals (1).
The training regimen in transplant patients must address overall conditioning and education. Aerobic exercises should be done at 60% to 70% of peak effort for 30 to 60 minutes 3 to 5 times weekly with rating of perceived exertion (RPE) targets, using the Borg scale, at 13 to 14, with the level of activity increasing incrementally to stay at this level. Education for posttransplant patients includes the complicated medical regimen, vocational, and psychological needs. The outcomes of rehabilitation in the cardiac transplant population have been generally favorable. Patients usually achieve increased work output and improved exercise tolerance, even resuming competitive athletics (1).
Cardiomyopathy
Despite not being covered by many insurance plans, CHF patients are a growing subset of the cardiac rehabilitation population. New Medicare regulations now will cover rehabilitation for CHF starting in March 2014, and it is hoped that many other insurance plans will follow suit. CHF patients have increased complications compared to coronary artery bypass graft (CABG) or post-MI population, with a higher risk of sudden death, depression, and chronic cardiac disability. They may also have inconsistent responses to exercise with increased fatigue and with possible exertional hypotension, as well as syncope. Low endurance and chronic fatigue are common, but may be improved with appropriate exercise. Since many CHF patients have very low exercise capacity, even a small improvement in VO2 can mean improved quality of life and even living independently for a patient with heart failure with ongoing rehabilitation (3).
Since CHF patients have higher risk than most cardiac rehabilitation patients, a graded ETT is essential before starting. Due to poor adaptation to exercise in CHF, long warm-up and cool-down periods are required with exercise at a limited workload. Dynamic exercise is preferable with a target HR at 10 bpm below any significant endpoint found with cardiopulmonary exercise testing. Isometric exercises need to be avoided since they increase diastolic pressure and cardiac afterload. Cardiac exercise should be started under supervision with cardiac monitoring initially. Patients with severe left ventricular dysfunction will need telemetry during warm-up, exercise, and cool-down. Once the patient can self-monitor, he or she can start a self-monitored program. CHF patients also need to closely follow body weight (to observe for fluid accumulation), and blood pressure and HR responses to exercise (3).
For more end-stage heart failure, management may include pharmacologic inotropic support or left ventricular mechanical support. Exercise can be done on intravenous inotropes with the same precautions as in other CHF patients (3). LVAD patients will usually follow the usual past surgical course, with phase 1 and 1B rehabilitation followed by phase 2 and 3 programs. Rehabilitation of LVAD in rehabilitation acute and subacute units requires a trained staff, close cooperation with the LVAD team, and familiarity with the devices that are used locally. Since CO is typically well sustained with the device, patients can have good exercise tolerance with the limit to peak exercise capacity limited by the peak flow of the device. Family and patient education are also essential parts of rehabilitation post LVAD (1).
Valvular Heart Disease
Cardiac rehabilitation for valvular heart disease is similar to rehabilitation for CHF. Postsurgical management is also similar to other postsurgical cardiac patients with the one issue of anticoagulation postoperatively for patients with mechanical valves. With anticoagulation, exercises need to avoid high-impact exercises to prevent hemarthroses and bruising and includes education regarding injury avoidance (3). The overall training program is similar to that discussed for the post-CABG patient (3).
Cardiac Arrhythmias
Patients with cardiac arrhythmias need closer telemetry monitoring for any change in intensity levels of exercise and new exercises. Patients identified as high risk of cardiac arrhythmias will need a monitored setting rather than a self-directed home program. For those patients with life-threatening arrhythmias, automatic implantable cardiac defibrillator (AICD) is commonly used. With AICD, exercise programs need to avoid the HR at which the device is set to fire. A pre-rehabilitation exercise stress test and cardiac precautions with target HR set well below the trigger threshold is a sufficient modification for the exercise program. As with other treatments, education and emotional support are an important part of the program (1).
Pulmonary Rehabilitation Programs in Specific Conditions
Emphysema
COPD rehabilitation is essentially standard pulmonary rehabilitation. The goals of the program are disease management and improvement of exercise capacity. Since the rehabilitation program cannot improve the function of the lungs, the goal is to allow for more work with the given ability to ventilate. Key aspects of the program are energy conservation education (how to do a given activity at a lower level of exertion) and improved endurance. The focus is on moderate-intensity exercises with longer duration rather than on high-intensity or short-burst exercises. Isometric exercises are not recommended due to increased intrathoracic pressures. Supplemental oxygen should be used as needed to maintain saturation above 90%, with education to return supplemental oxygen to baseline resting need after exercise to prevent resting hypercarbia. Patients with COPD generally have relatively modest oxygen needs and can often exercise with 1 to 6 L of oxygen via nasal cannula. For patients with sleep apnea or ventilatory failure, programs should also concentrate on incorporation of the bilevel ventilation into their routine and look to improve compliance. Of special note, for patients being considered for LVRS, pulmonary rehabilitation is considered essential both to qualify for the surgery and after surgery to assure adequate outcomes (17).
For patients with significant secretions, airway clearance and chest physical therapy may be an important part of the rehabilitation program. External percussion devices, vibration devices, and inhalation of saline may help to mobilize secretions. Cough training and huffing can help to clear secretions, and family training is essential Finally, medication education is essential, including the appropriate use of inhaled medications and oxygen and management of equipment (18).
Interstitial Lung Disease
Pulmonary rehabilitation for ILD is similar to that for obstructive lung disease. The key differences lie in that patients with ILD often have profound hypoxemia and need to have high-flow oxygen with exercise to maintain adequate saturation for activity. Prevention of chronic hypoxemia is essential to preventing the onset of PH as this secondary pulmonary disease, when present with ILD, makes patients profoundly symptomatic and can markedly decrease life expectancy. Appropriate exercise can be more intense in this group of patients, with oxygenation the key limiting factor. Airway clearance is generally less of an issue and most patients with ILD are not on ventilatory support until the very end stages of disease when rehabilitation may no longer be possible (19).
Since many of these conditions are progressive, it is often important to either have patients referred for transplantation or start end-of-life planning to allow for achieving as many patient goals as possible.
Pulmonary Hypertension
Patients with PH are very similar to CHF patients with many similar precautions. With the onset of effective pulmonary vasodilators, patients now have much longer life expectancy and live with chronic management for decades. Because they may have experienced debility prior to the institution of effective therapy or have concern about exercise many patients could benefit from pulmonary rehabilitation. It is important to maintain oxygenation during exercise, and patients with severe PH may need to have cardiac monitoring as arrhythmias and right heart failure are issues to be considered. High-flow supplemental oxygen and education in the use of their vasodilating agents are part of the program. Patients with intravenous or continuous subcutaneous vasodilating medication infusions can be safely incorporated into exercise programs, but there need to be long warm-up and cool-down periods with an emphasis on moderate- to low-level exercise for patients with severe pulmonary vascular disease. Further research into efficacy and safety of pulmonary rehabilitation for patients with pulmonary vascular disease is still ongoing.
Ventilatory Failure
Patients with ventilatory failure who are on either invasive or noninvasive ventilation require mobilization as well and should have exercise programs. For patients with nocturnal or intermittent ventilatory support, exercise programs can improve efficiency and allow greater activity and less fatigue while off the ventilator. Detailed management for patients with need for noninvasive ventilation is beyond the scope of this chapter. Table 11.8 has an overview of the types of patients who may present with ventilatory failure.
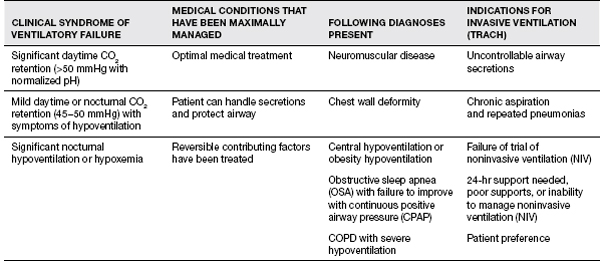
A summary of the indications for ventilatory support is in Table 11.9 (8).
Cardiopulmonary Rehabilitation in the Physically Disabled
Finally, there are some special considerations for patients with both disability and cardiac disease. Most of the difficulties in cardiac rehabilitation in this population are due to limited mobility, which presents difficulty in both testing and exercise training. Patients with disability may also be at higher risk of cardiac and pulmonary disease, and the presence of cardiopulmonary limitations should be remembered when engaging in any standard rehabilitation program. Patients with stroke or peripheral vascular disease are at particularly high risk since these conditions often have concurrent cardiac disease, but any patient with disability may have a cardiac or pulmonary comorbidity. In cases where cardiac or pulmonary disease is overt, cardiopulmonary rehabilitation should be provided for these disabled individuals just as it would be for the able-bodied population. Cardiopulmonary primary and secondary prevention is also important for patients with physical disabilities as they are usually more sedentary and have high rates of obesity and deconditioning. Additionally, disabled individuals usually require higher energy expenditures for mobility, with a resultant need for increased work capacity and potentially more disability from a similar level of cardiopulmonary disease.
When prescribing cardiopulmonary exercise for disabled individuals, the exercise protocols need to be adapted for the individual patient. Patients with lower extremity impairment due to neurological or orthopedic conditions can perform upper extremity ergometry, and modification of lower extremity exercise equipment will allow them to exercise with their legs. Hemiplegic patients can use adapted bicycle ergometers or airdynes. Since exercise protocols for stroke and other conditions incorporate upper limb exercise, the high MVO2 requirements for upper extremity exercise should be considered when designing a cardiac rehabilitation program for disabled patients. It is essential for disabled patients to focus particularly on task-specific activities in order to improve aerobic conditioning and endurance, while seeking to lower MVO2 needed with each task. The physiatrist is particularly suited to take a leadership role in the area of the design of cardiopulmonary rehabilitation programs for the disabled since most traditional cardiac rehabilitation programs have limited experience with the needs of physically disabled patients.
TABLE 11.8 Assessment of Ventilatory Failure
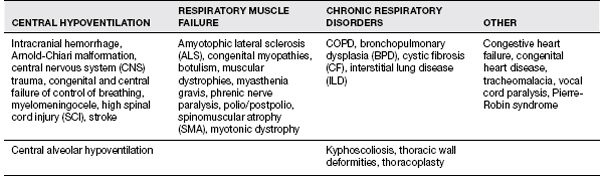
TABLE 11.9 The Indications for Ventilatory Support
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



