The goals of burn wound care are removal of nonviable tissue, prevention of infection, and facilitation of wound healing, while controlling pain and maximizing outcome. This article reviews the basic pathophysiology of burn wounds; describes the evaluation of the depth, location, and extent of the wound; and discusses the myriad of wound care products on the market including their strengths and weaknesses. This article guides the reader through wound assessment and designing the appropriate treatment plan.
The goals of burn wound care are removal of nonviable tissue, prevention of infection, and facilitation of wound healing, while controlling pain and maximizing outcome. This article reviews the basic pathophysiology of burn wounds; describes the evaluation of the depth, location, and extent of the wound; and discusses the myriad of wound care products on the market including their strengths and weaknesses. A burn clinician must remember that they are treating patients and not wounds and that adapting to the patient’s individual circumstances is essential, including the type of wound and access to resources (ie, someone to change the dressing, a wound care product they can find and afford, and so forth). This article guides the reader through wound assessment and designing the appropriate treatment plan.
Initial assessment
Burns cannot be managed without a direct visualization of the wound bed. Patients must be undressed and dressings must be removed. Even with direct visualization, it is quite difficult to determine wound depth initially. Many an expert clinician has been fooled on the initial evaluation. Multiple technologies have attempted to circumvent this challenge including indocyanine green fluorescence, histologic assessment, and Doppler technology. Unfortunately, these are not cost effective and have no more accuracy than the expert clinician. One of the ways around this is to have a good understanding of the mechanism of burn injury and the duration of hot contact, which can help determine the depth. In general, the hotter the substance and the greater the duration of contact, the deeper the burn. With a water temperature of 120°F, it takes in the range of 30 seconds to 10 minutes to obtain a full-thickness burn. With a temperature of 140°F to 150°F, it only takes a few seconds. Water splash burns are not as deep as cheese sauce, which is not as deep as tar. Things that stick and dissipate heat into the skin create a deeper wound. Burns in patients with absent or decreased sensation tend to be full-thickness despite how they may appear initially. The clinician must also be concerned if the pattern, depth, and mechanism of injury do not match. This is especially true for children, those with cognitive impairment, and the elderly. A story of a spill burn in a child with a water line mark consistent with being held under hot water requires referral to the appropriate authorities for investigation.
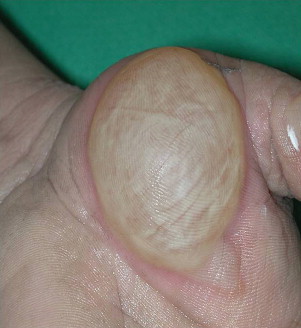
Wound assessment is based on color, degree of epithelial separation, and the coagulation of the eschar. Superficial (first degree) burns affect only the epidermis. This is essentially the depth of most sunburn. Individuals present with moderate pain and erythema without blistering. The redness resolves over a few days without any epithelial loss. Partial-thickness burns (second degree) involve the epidermis and part of the dermis. Superficial partial thickness is sunburn that peels. It heals without scarring within a week. Mid partial-thickness burns effect about half of the dermal layer. This is a burn that blisters initially. There are numerous extensive reviews of whether these blisters should be unroofed or not ( Fig. 1 ). Leaving a blister intact decreases pain but may increase infection risk. In young children, it may make sense to leave the blister intact for the first few days to avoid discomfort. If the blister is opened by the clinician or if it opens spontaneously, an attempt can be made to get the separated epithelium to stick to the wound bed and act like a biologic dressing. As the wound epithelizes, the necrotic epithelium lifts off. This is an ideal way to limit pain and facilitate healing. This depth of burn takes about 2 weeks to heal and usually does not produce scarring but may result in pigmentation changes.
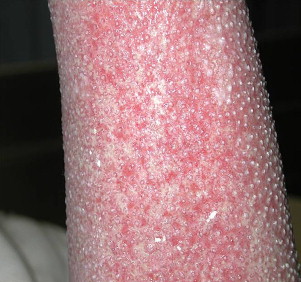
Deep partial-thickness burns involve the entire epidermis and dermis but spare the base of the hair follicle, which is lined with epithelial cells. It presents initially with nonadherent eschar. Once it is fully clean, numerous skin buds are visible ( Fig. 2 ). This depth of burn is the most painful wound seen because of the exposed nerve endings associated with the skin buds. Although it heals spontaneously over about 3 weeks, the chaotic organization of the dermal matrix may lead to significant hypertrophic scarring.
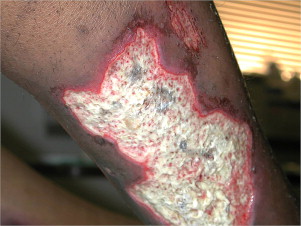
Full-thickness (third degree) burns present with a leathery appearance with well demarcated wound edges ( Fig. 3 ). Hair, when pulled, slides out; thrombosed capillaries may be seen; and it has an adherent appearance. In general, a burn of this depth that is larger than the size of a quarter or in an area of important cosmesis or function, such as the back of the hand, requires skin grafting. These burns are mostly insensate because the nerve endings in the dermal layer have been lost but the clinician must remember that most burns are not a uniform depth and may have some areas of sparing of the nerve endings that can cause exquisite pain. Deep full-thickness (fourth degree) burns involve tendon, muscle, or bone. These wounds present with a charred or mummified appearance and amputation is universally required.
Pathophysiology
The burn wound can be divided into several zones. The central part of the wound, which had the most contact with the heat source, is the zone of coagulation in which cells are irreversibly damaged with no potential for regeneration. Immediately adjacent is the zone of stasis in which the cells have decreased blood flow but may be salvageable with fluid resuscitation and delicate wound care. Further ischemia or infection results in irreversible loss of this layer. The final zone is the zone of hyperemia. In this area, the cells have been inflamed but are not necrotic. The skin of these areas may look pink, which makes it difficult to determine if a patient has cellulitis. Marking these areas to look for progression over time may help with this determination. In response to the burn injury, the disrupted blood vessels in the outer two zones have increased capillary permeability, which results in water, protein, and electrolyte leak and edema formation. With circumferential injury on the chest or limbs, this pressure can create a tourniquet effect with resultant ischemia so close monitoring of tissue pressures may be warranted. This fluid loss continues until the skin surface has reepithelized. The fluid loss with resultant hemoconcentration can lead to capillary stasis and further ischemia of the wound bed.
Pathophysiology
The burn wound can be divided into several zones. The central part of the wound, which had the most contact with the heat source, is the zone of coagulation in which cells are irreversibly damaged with no potential for regeneration. Immediately adjacent is the zone of stasis in which the cells have decreased blood flow but may be salvageable with fluid resuscitation and delicate wound care. Further ischemia or infection results in irreversible loss of this layer. The final zone is the zone of hyperemia. In this area, the cells have been inflamed but are not necrotic. The skin of these areas may look pink, which makes it difficult to determine if a patient has cellulitis. Marking these areas to look for progression over time may help with this determination. In response to the burn injury, the disrupted blood vessels in the outer two zones have increased capillary permeability, which results in water, protein, and electrolyte leak and edema formation. With circumferential injury on the chest or limbs, this pressure can create a tourniquet effect with resultant ischemia so close monitoring of tissue pressures may be warranted. This fluid loss continues until the skin surface has reepithelized. The fluid loss with resultant hemoconcentration can lead to capillary stasis and further ischemia of the wound bed.
Initial wound care
Once the initial assessment has been completed, the wound needs to be cleaned thoroughly. Twenty years ago this wound care was done in a hydrotherapy tank. This language is still used today in the terms “hydro,” “tanking,” and “bathing” even though lowering patients into a tub of water is almost never done. Although many patients preferred the warmth of soaking, it was clear over time that the risks outweighed any potential benefits. Putting patients in water can result in hypothermia even if the water is relatively warm. Wound cross-contamination from one site to another can occur. Because, the fluid leak continues, it may worsen underlying hyponatremia. Lastly, and probably the final straw, was that reimbursement for “tanking” is the same as for local care and the cost of filling, emptying, and cleaning the tanks made it fiscally impossible to continue this practice.
Modern wound care should be done in a designated area where strict infection prevention and control practices are mandated. The wound is washed with soap and water. Antibacterial soap and sterile water are not required. Using sterile technique the wounds are rinsed and scrubbed. The technique for rinsing can be gentle pouring of water over the wound or using a spray hose. Pulse lavage is painful and may over-debride, so is no longer commonly used. All topical agents and necrotic tissue should be removed. Often times, a straight edged razor, scissors, or forceps are required. In a facility, as much nonviable tissue as possible should be removed in each session. For patients doing their wound care at home a slower approach may be the pragmatic answer, removing only tissue that has clearly separated, because it takes an experienced professional to clearly differentiate viable from nonviable tissue, particularly in a wound of mixed depth.
Topical agents
Once the wound bed is clean the debate over the best topical agent or dressing begins. Unfortunately, there are actually relatively few good studies of the topical treatments of burn wounds. Silver sulfadiazine (Silvadene; King Pharmaceuticals, Bristol, TN, USA) has been the mainstay of burn care for more than 40 years. Although there are many other wound care products on the market, a recent survey shows that most burn care experts continue to use silver sulfadiazine. Wound healing may be a day or two slower with silver sulfadiazine compared with other agents. Although sulfur allergy is a relative contraindication for silver sulfadiazine, most patients with a reaction to oral sulfonamides tolerate it without difficulty. An occasional patient has a stinging sensation with silver sulfadiazine and those individuals should be switched to an alternative dressing. Glucose-6-phosphate dehydrogenase deficiency is a contraindication, because hemolysis can occur in these individuals. Silver sulfadiazine can be applied with a tongue blade to the thickness of butter on bread ( Fig. 4 ). Dressings with silver sulfadiazine can stick to the wound bed, but this can be prevented by lightly impregnating the gauze with silver sulfadiazine at the time of application. If the dressing does stick, thoroughly wetting the dressing facilitates removal. Patients complain of slightly more pain than with other agents. Although there was a theoretical concern about neutropenia, a large scale study showed no decrease in white blood counts in patients who received prolonged treatment with silver sulfadiazine. Compared with other agents, the depth of penetration is less than ideal. It is bacteriostatic and it may impair epithelialization. So why do burn care providers continue to use silver sulfadiazine? It is widely available even in rural pharmacies; it is easy to apply; and it is generally soothing, particularly to exposed skin buds. Perhaps the main issue, however, is cost. At my own institution we use 2 tons of silver sulfadiazine per year to treat over 600 inpatients and more than a 1000 outpatients. That is 4000 lbs = 8800 kg. Our wholesale cost for silver sulfadiazine is $11 for a 400 g jar. That means we spend $2.42 million on silver sulfadiazine per year. If we switch to a wound care product that is $11 for 40 g then our costs go up $18 million dollars. Therefore, like other centers, we continue to use silver sulfadiazine for most wounds.
The second most common wound care agent selected by burn experts is antibiotic-impregnated gauze. Although these products have also been shown to slightly slow time to full healing compared with other agents, they are readily available and easy to use. Triple antibiotic is available in almost any general store and is inexpensive and easy to use. These dressings have an even greater tendency to stick to the wound bed, which may damage newly healed epithelial cells. For large burns, single-ingredient antibiotic ointments are preferred to avoid the increase risk of allergic reaction associated with the use of a triple antibiotic ointment. For most burn units this agent is bacitracin. Mupirocin is usually reserved for treating patients with methicillin-resistant Staphylococcus aureus (MRSA).
Mafenide acetate (Sulfamylon; Bertek Pharmaceuticals, Morgantown, WV, USA) has been commonly used in burns for many years. It has an excellent depth of penetration and provides broad-spectrum antimicrobial coverage but is not readily available outside of the burn unit and may produce a stinging sensation when used. The 5% solution is used on infected wounds or for very deep wounds. Mafenide acetate cream is the agent of choice for full-thickness burns of the ears. It is somewhat expensive but the cost can be offset in areas that use large volumes.
Acetic acid (0.25% solution) has been used in burn care. It is useful as a soak to decrease colonization with Pseudomonas but is usually not left on the wound because of excessive dryness. Domboro solution (Bayer, Morristown, NJ, USA) can also be used in a similar way for controlling overgrowth of Pseudomonas .
Biologic dressings
Biologic dressings are temporary dressings that can decrease pain and fluid loss and facilitate wound closure. Xenograft (pigskin) is inexpensive and comes in large sheets. It is ideal for use over clean midpartial thickness wounds, such as chest scald burns in children ( Fig. 5 ). It is left dry and eliminates the need for dressing changes. The healing epithelium pushes the xenograft off leaving healed skin underneath. As it dries, it can give a pulling or tight sensation. If left on for more than 2 weeks, it can get incorporated into the skin and create a granulomatous phenomenon. If it is still adherent after 2 weeks, it can be coated with a thick layer of silver sulfadiazine, wrapped in gauze, and left overnight. The following day, the adherent xenograft separates off with soaking. Allograft (cadaveric skin) can be used on any partial-thickness burn but because of expense and the small size of the pieces, it is primarily reserved for chronic nonhealing wounds or to assess the viability of a full-thickness wound bed for accepting autograft. Although it may remain in place for weeks, it is only a temporary coverage and must eventually be replaced with autograft. Autograft is the permanent coverage for large full-thickness wounds. Small wounds may close by epithelizing from the wound edges (see the article on acute care elsewhere in this issue).









