This article reviews the recent literature regarding bone health as it relates to the patient living with neuromuscular disease (NMD). Studies defining the scope of bone-related disease in NMD are scant. The available evidence is discussed, focusing on abnormal calcium metabolism, increased fracture risk, and the prevalence of both scoliosis and hypovitaminosis D in Duchenne muscular dystrophy, amyotrophic lateral sclerosis, and spinal muscular atrophy. Future directions are discussed, including the urgent need for studies both to determine the nature and extent of poor bone health, and to evaluate the therapeutic effect of available osteoporosis treatments in patients with NMD.
- •
Poor bone health is common in patients with neuromuscular disease and is the cause of significant morbidity, including increased fracture rates and severe scoliosis.
- •
Bone health depends on a complex interplay of both local and distant mechanisms, including genetic, endocrine, neurologic, and lifestyle factors.
- •
Osteoporosis in neuromuscular disease may be due to disease-specific pathophysiology, but appears to frequently be complicated by hypovitaminosis D with osteomalacia, as evidenced by incomplete improvements in bone density when serum vitamin D is replete.
- •
The use of glucocorticoids in Duchenne muscular dystrophy extends independent ambulation; however, its effects on bone health have not been completely studied, and may have adverse effects on bone density and increase fracture risk. Further studies are warranted.
- •
Further research is needed to assess the extent of poor bone health across all neuromuscular disease and to evaluate the efficacy of known osteoporosis treatments in this unique patient population.
Introduction
The Bone and Joint Decade, an international collaborative movement sanctioned by the United Nations and World Health Organization, has focused worldwide attention on the growing burden of musculoskeletal and bone disease. In the United States alone, it is estimated that more than 1 in 4 people will require treatment for a musculoskeletal disorder. Jacobs and colleagues reported that during 2004, the United States spent $849 billion in direct and indirect costs toward bone and joint health, equaling 7.7% of the gross domestic product. As a direct consequence of highlighting this growing public health concern, the Bone and Joint Initiative has increased research focused on unraveling the basic biological mechanisms involved in bone development and maintenance of bone health. Although the literature regarding musculoskeletal and bone disorders in neuromuscular disease (NMD) remains scant, the research advances generated as a result of this movement are relevant to and provide insight for therapeutic approaches. Poor bone health is often a significant problem for patients with NMD. Deficiency of bone mineral density (BMD) and increased incidence of bone fractures, for example, are a well-recognized clinical consequence of diseases such as Duchenne muscular dystrophy (DMD), amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA). A long bone fracture in a patient with NMD often heralds loss of independent ambulation. Furthermore, therapy with corticosteroids, a recommended treatment for DMD, may have deleterious effects on bone health, which has not been extensively explored. The aim of this review is to present the current literature on bone development and health as it relates to the patient diagnosed with an NMD, and to demonstrate the need for disease-specific research to develop both diagnostic and therapeutic treatment strategies aimed at improving bone health and reducing associated morbidity in this population at risk.
Normal bone growth and development
Bone Components and Formation
The skeleton is a dynamic, metabolically active organ that is in constant flux. Our bones serve 2 main functions: a metabolic function, as the reservoir for calcium and phosphate needed for serum homeostasis; and a structural function, housing and protecting vital organs and serving as a strut for muscle attachment, which permits locomotion. There are 2 types of bone in the normal, mature human skeleton: cortical and trabecular. Although macroscopically and microscopically different, the 2 forms are identical in their chemical composition. Cortical bone is dense and compact, has a slow turnover rate with high resistance to bending and torsion, and constitutes the outer layer of the bony structure. Trabecular bone is less dense, more elastic, contributes to mechanical support particularly in bones such as the vertebrae, and provides the initial supply of minerals in acute deficiency states. In osteoporosis, a disease characterized by reduced bone strength and increased susceptibility to fractures, trabecular bone is often more severely affected than cortical bone.
The structural components of bone consist of a largely mineralized extracellular matrix, constructed of type I collagen fibers and noncollagenous proteins. The matrix accounts for approximately 90% of the organic composition of the skeleton. The most abundant noncollagenous matrix protein is osteocalcin. Osteocalcin participates in the stabilization of the hydroxyapatite matrix and binds calcium. It is a negative regulator of bone formation and inhibits premature or inappropriate mineralization. By contrast, biglycan, another noncollagenous bone matrix protein, positively regulates bone formation.
Bone Growth
Total skeletal calcium increases from approximately 25 g at birth to 1200 g in early adulthood. These gains are achieved through bone modeling, the process that alters bone length, diameter, and shape during growth. The cells responsible for osteogenesis (the embryonic or postfracture process of bone formation), bone modeling, and bone remodeling are the osteoblast, osteocyte, and osteoclast. Osteoblasts are small, single-nucleated cells that lay new bone distant from the resorption site and line all bone surfaces as “lining cells.” As osteoblasts encapsulate themselves in bone matrix during modeling and remodeling, they become quiescent and are then considered osteocytes. Osteoclasts are large multinucleated cells that resorb old or damaged bone beneath the periosteum by acidification and proteolysis of the bone matrix and hydroxyapatite crystals.
Most of the accrual in bone mineral content during growth is due to increases in bone size, rather than density. However, in contrast to cortical bone density, which remains constant across age, gender, and race, trabecular bone increases in density during puberty. The increases in trabecular bone density have been observed in the lumbar spine. Once growth ceases, aged bone is continuously replaced through the process of remodeling, which serves to repair microdamage and maintain skeletal integrity without altering bone size or shape.
Bone Remodeling
Bone remodeling is accomplished by groups of osteoblasts and osteoclasts acting together in concert. This functional group is referred to as the basic multicellular unit. The process of remodeling is thought to be activated by osteoblast lineage cells including the lining cells, mesenchymal stem cells located within bone marrow, and osteocytes. Evidence suggests that osteocytes are the primary mediator of the remodeling cycle. However, each of these cells secrete receptor activator nuclear factor κB ligand (RANKL), a protein belonging to the tumor necrosis factor (TNF) superfamily, which initiates fusion of osteoclast lineage cells producing mature osteoclasts. Osteoclast precursor cell fusion occurs through the interaction of RANKL and RANK, an osteocyte lineage cell surface binding site. The processes leading to cell fusion are inhibited by both osteoprotegerin (OPG), a dimeric glycoprotein that functions as a decoy receptor and blocks the RANKL-RANK interaction, and sclerostin, a glycoprotein antagonist of the Wnt signaling pathway. OPG and sclerostin are mainly produced and secreted by osteoblast lineage cells. The OPG-RANKL-RANK interactions illustrate mechanisms of local control and coupling of bone formation and resorption cycles.
In children, bone formation typically outpaces resorption, whereas in the young adult bone formation is coupled to resorption. With aging and in many pathologic bone conditions, resorption shifts to exceed formation, resulting in a negative bone balance and loss of BMD.
Growth, development, and maintenance of healthy bone are controlled by a complex multifactorial process with both local and distant regulation including genetic, endocrine, neurologic, and lifestyle influences. Disturbances in any component of this well-integrated process may cause marked alterations in bone modeling and remodeling, often resulting in abnormal bone density and increased risk of fracture.
Normal bone growth and development
Bone Components and Formation
The skeleton is a dynamic, metabolically active organ that is in constant flux. Our bones serve 2 main functions: a metabolic function, as the reservoir for calcium and phosphate needed for serum homeostasis; and a structural function, housing and protecting vital organs and serving as a strut for muscle attachment, which permits locomotion. There are 2 types of bone in the normal, mature human skeleton: cortical and trabecular. Although macroscopically and microscopically different, the 2 forms are identical in their chemical composition. Cortical bone is dense and compact, has a slow turnover rate with high resistance to bending and torsion, and constitutes the outer layer of the bony structure. Trabecular bone is less dense, more elastic, contributes to mechanical support particularly in bones such as the vertebrae, and provides the initial supply of minerals in acute deficiency states. In osteoporosis, a disease characterized by reduced bone strength and increased susceptibility to fractures, trabecular bone is often more severely affected than cortical bone.
The structural components of bone consist of a largely mineralized extracellular matrix, constructed of type I collagen fibers and noncollagenous proteins. The matrix accounts for approximately 90% of the organic composition of the skeleton. The most abundant noncollagenous matrix protein is osteocalcin. Osteocalcin participates in the stabilization of the hydroxyapatite matrix and binds calcium. It is a negative regulator of bone formation and inhibits premature or inappropriate mineralization. By contrast, biglycan, another noncollagenous bone matrix protein, positively regulates bone formation.
Bone Growth
Total skeletal calcium increases from approximately 25 g at birth to 1200 g in early adulthood. These gains are achieved through bone modeling, the process that alters bone length, diameter, and shape during growth. The cells responsible for osteogenesis (the embryonic or postfracture process of bone formation), bone modeling, and bone remodeling are the osteoblast, osteocyte, and osteoclast. Osteoblasts are small, single-nucleated cells that lay new bone distant from the resorption site and line all bone surfaces as “lining cells.” As osteoblasts encapsulate themselves in bone matrix during modeling and remodeling, they become quiescent and are then considered osteocytes. Osteoclasts are large multinucleated cells that resorb old or damaged bone beneath the periosteum by acidification and proteolysis of the bone matrix and hydroxyapatite crystals.
Most of the accrual in bone mineral content during growth is due to increases in bone size, rather than density. However, in contrast to cortical bone density, which remains constant across age, gender, and race, trabecular bone increases in density during puberty. The increases in trabecular bone density have been observed in the lumbar spine. Once growth ceases, aged bone is continuously replaced through the process of remodeling, which serves to repair microdamage and maintain skeletal integrity without altering bone size or shape.
Bone Remodeling
Bone remodeling is accomplished by groups of osteoblasts and osteoclasts acting together in concert. This functional group is referred to as the basic multicellular unit. The process of remodeling is thought to be activated by osteoblast lineage cells including the lining cells, mesenchymal stem cells located within bone marrow, and osteocytes. Evidence suggests that osteocytes are the primary mediator of the remodeling cycle. However, each of these cells secrete receptor activator nuclear factor κB ligand (RANKL), a protein belonging to the tumor necrosis factor (TNF) superfamily, which initiates fusion of osteoclast lineage cells producing mature osteoclasts. Osteoclast precursor cell fusion occurs through the interaction of RANKL and RANK, an osteocyte lineage cell surface binding site. The processes leading to cell fusion are inhibited by both osteoprotegerin (OPG), a dimeric glycoprotein that functions as a decoy receptor and blocks the RANKL-RANK interaction, and sclerostin, a glycoprotein antagonist of the Wnt signaling pathway. OPG and sclerostin are mainly produced and secreted by osteoblast lineage cells. The OPG-RANKL-RANK interactions illustrate mechanisms of local control and coupling of bone formation and resorption cycles.
In children, bone formation typically outpaces resorption, whereas in the young adult bone formation is coupled to resorption. With aging and in many pathologic bone conditions, resorption shifts to exceed formation, resulting in a negative bone balance and loss of BMD.
Growth, development, and maintenance of healthy bone are controlled by a complex multifactorial process with both local and distant regulation including genetic, endocrine, neurologic, and lifestyle influences. Disturbances in any component of this well-integrated process may cause marked alterations in bone modeling and remodeling, often resulting in abnormal bone density and increased risk of fracture.
Determinants of bone mass
A brief overview of selected aspects of the physiologic processes involved in bone acquisition and maintenance of bone density is presented here. Many of these processes are likely to be disturbed in patients with NMD and may suggest some rationales for treatments aimed at improving bone health.
Genetic Factors
Numerous factors are important in influencing the achievement of maximum bone height and density. However, bone size potential for an individual, as defined as the size a bone can reach under optimal circumstances, is determined by genetic factors. There have been considerable advances made over the past decade toward understanding the genetic basis of bone development and maintenance. However, the scope of this review encompasses the introduction of a signaling pathway necessary for normal bone metabolism. The interested reader is encouraged to read the review by Karsenty and colleagues, which covers the topic in greater depth.
Wnts are an evolutionarily conserved family of growth factors whose signaling is involved in numerous processes, including bone formation and maintenance. The Wnt/β-catenin pathway plays a crucial role in bone formation and generally promotes an increase in bone mass by mechanisms including renewal of stem cells, osteoblast proliferation, induction of osteoblast formation, and inhibition of osteoblast and osteocyte apoptosis.
Endocrine Factors
Parathyroid hormone
Parathyroid hormone (PTH) is released from chief cells in the parathyroid glands when the plasma calcium concentration decreases, acting as the key regulator of calcium and phosphate homeostasis. The direct actions of PTH on kidney and bone, or the indirect actions on the intestines contribute to restoring the concentration of plasma calcium. These responses are mediated by parathyroid receptors that bind PTH. The response of bone depends on interactions between osteoblasts and osteoclasts, as only osteoblasts express the parathyroid receptor, and is variable depending on PTH secretion patterns. In experimental models of osteoporosis in which bone loss was induced by ovariectomy, intermittent treatment with PTH led to increased osteoblastic activity, with recovery of bone density. When secreted continuously, however, PTH induced osteoblast-osteoclast coupling factors, promoting resorption by increasing secretion of RANKL.
PTH is the first anabolic drug to be approved for the treatment of osteoporosis. Tu and colleagues recently reported results from a small prospective trial in which patients with a history of multiple osteoporotic vertebral compression fractures were followed. None of the 28 patients treated with PTH, over a period of at least 18 months, experienced new-onset vertebral fracture, and vertebral bone density increased.
Calcitonin
Calcitonin is produced by the parafollicular C cells of the thyroid gland. High levels of plasma calcium stimulate secretion of calcitonin, which activates renal calcium excretion and impairs osteoclast function. The downstream effects, mediated by calcitonin receptors found on osteoclasts, promote bone formation by reducing osteoclast motility, bone-surface binding, and proteolytic enzyme secretion. Calcitonin nasal spray has demonstrated the ability to decrease fracture risk and has been approved by the Food and Drug Administration for the treatment of osteoporosis since 1995. Oral preparations have been in development; however, after an initial successful 3-month phase 2 multicenter, randomized, double-blind, placebo-controlled dose-ranging trial demonstrating reduced serum and urine bone turnover markers (BTMs), Novartis announced in late 2011 that their 3-year phase 3 multicenter trial did not produce significant reductions in vertebral or nonvertebral fracture risk.
Androgen hormones
Androgen hormones are necessary for normal growth and maintenance of bone health. Androgen receptors are ubiquitously expressed across bone cell types. The role that androgens play in bone modeling has been well explored in animal and human studies over the last 3 decades. Testosterone increases skeletal calcium uptake in prepubertal boys, and testosterone therapy has been shown in both prospective and retrospective studies of male hypogonadism to increase bone density. Androgens influence longitudinal bone growth during early puberty and epiphyseal growth-plate closure in later puberty by direct effects on growth-plate chondrocytes. Under strict culture conditions, Carrascosa and colleagues demonstrated that androgens regulate both proliferation and differentiation of cultured epiphyseal chondrocytes. In addition, androgens appear to have indirect effects on pituitary function, shifting the kinetics of growth-hormone secretion during puberty.
Estrogen hormones
Estrogen receptors are expressed within the human growth plate. Studies have confirmed that epiphyseal closure during late puberty depends on estrogen in both males and females. Estrogens appear to play a greater role than testosterone in preventing bone loss in elderly men, and testosterone’s effects may be indirect and mediated through the estrogen receptor, as testosterone is metabolized via the cytochrome P450 aromatase enzyme complex into 17β-estradiol. In one uncontrolled study of eugonadal men, Anderson and colleagues showed that testosterone therapy appeared to exert its beneficial effects mainly through increased levels of serum estrogen, as estrogen levels increased more than serum testosterone levels.
Estrogen decreases the responsiveness of osteoclast progenitor cells to RANKL, preventing osteoclast formation and shortening the life span of osteoclasts. Estrogen affects genes coding for enzymes, bone-matrix proteins, hormone receptors, and transcription factors. Estrogen also upregulates the production of OPG, insulin-like growth factors, and tissue growth factor β, promoting bone formation.
Glucocorticoids
The role of glucocorticoids in bone health and disease is complex, with both stimulatory and inhibitory effects on bone cells. Glucocorticoids are important for the normal regulation of bone remodeling and are essential for osteoblast differentiation from mesenchymal stem cells. Glucocorticoids influence osteoblast gene expression, including downregulation of type I collagen and osteocalcin, and upregulation of interstitial collagenase. The synthesis of osteoblast growth factors are modulated by glucocorticoids. For example, the expression of insulin-like growth factor I, an important osteoblast trophic factor, is decreased by glucocorticoids.
Glucocorticoids can have varying and quite opposing effects on bone. Whereas endogenous glucocorticoids at appropriate physiologic levels are necessary for normal bone health and development, abnormally increased endogenous secretion or pharmacologic glucocorticoids induce bone loss and promote osteoporosis. Prolonged exposure to excess glucocorticoids is the most common cause of secondary osteoporosis. Clinically, patients with glucocorticoid-induced osteoporosis develop bone loss within the first few months of glucocorticoid exposure. It has been reported that bone loss occurs with a rapid phase of about 12% within the first year of glucocorticoid administration, followed by a slow phase of 2% to 5% annually. Multiple practice guidelines have been written recommending treatment protocols for patients, including those diagnosed with DMD who are placed on chronic glucocorticoid treatment, to reduce fractures and the morbidity associated with glucocorticoid-induced osteoporosis ( Tables 1 and 2 ).
| Screening Categories | American College of Rheumatology | DMD Care Consideration |
|---|---|---|
| Bone density | Consider serial BMD testing | Annual BMD testing |
| Serum 25-hydroxyvitamin D | Consider annual serum 25-hydroxyvitamin D screening | Annual 25-hydroxyvitamin D screening in late winter |
| Height | Annual height measurement | Height screening every 6 mo |
| Fracture | Assessment of incident fragility fracture | Take a careful fracture history |
| Medication compliance | Assessment of osteoporosis medication compliance | No recommendation |
| Glucocorticoid treatment side effects | No recommendation | Screen for additional side effects with regular follow-up |
| Treatment Category | American College of Rheumatology | DMD Care Considerations |
|---|---|---|
| Vitamin D | Supplement vitamin D | Supplement with vitamin D 3 if serum level is less than 32 nmol/L: If between 20 and 31 nmol/L give 1000 IU orally twice daily If less than 20 nmol/L give 2000 IU orally twice daily Recheck serum 25-hydroxyvitamin D after 3 mo of treatment |
| Calcium | Calcium intake 1200–1500 mg/d | No recommendation |
| Physical activity | Encourage weight-bearing activities | Encourage weight-bearing activities |
| Lifestyle modification | Avoid: tobacco, alcohol >2 drinks per day | No recommendation |
| Osteoporosis medications | If glucocorticoid dose 7.5 mg/d or more and treatment will be at least 3 mo: add alendronate, risedronate, or zoledronic acid If high fracture risk, may treat with teriparatide | Consider treatment with a bisphosphonate, such as pamidronate |
Thyroid hormones
Thyroid hormones are required for skeletal development and the establishment of peak bone mass. Population studies indicate that both hypothyroidism and hyperthyroidism are associated with increased fracture risk. Growth retardation and delayed skeletal development occur in children who are hypothyroid. Hyperthyroidism increases renal excretion of calcium and phosphorus, resulting in bone loss. Therefore, maintaining a euthyroid state is essential for bone health. The processes by which thyroid hormone alters bone metabolism are not fully understood; however, increasing evidence exists to suggest a dependent interplay between thyroid hormone, insulin-like growth factor I, and the Wtn/β-catenin signaling pathway.
Neurologic Factors
Neurons and neurotransmitters are intimately involved in bone remodeling. Bones have abundant innervation with nerve processes running along vessels adjacent to bone trabeculae, where terminal nerve boutons are in contact with bone cells. Discovery of β2 adrenergic receptors and receptors for neurotransmitters such as glutamate and Neuromedin U, on both osteoblasts and osteoclasts, suggests a critical homeostatic role of the peripheral nervous system in the regulation of bone metabolism. In addition, central nervous system influence on bone metabolism has been linked to the hypothalamus through a Leptin-β2 adrenergic receptor–dependent system. Leptin is a 16-kDa peptide hormone synthesized by adipocytes, which affects appetite and energy metabolism through its binding to the leptin receptor located in the hypothalamus. Mice lacking a functional Leptin receptor are obese and sterile, and despite hypogonadism, the most common cause of osteoporosis, have high bone mass. The central signals mediated by Leptin are relayed through the sympathetic nervous system and target osteoblasts expressing β2 adrenergic receptors. In support of these findings, mice treated with isoproterenol, a β agonist, displayed a massive decrease in bone mass. Conversely, mice with blocked sympathetic nervous system signaling had high bone mass. β2 Adrenergic receptors also control osteoblast expression of both RANKL and mRNA for factors that promote bone resorption.
Ma and colleagues recently demonstrated that dexamethasone, a glucocorticoid, stimulates the expression of β2 adrenergic receptors in differentiated primary calvarial osteoblasts after short-term treatment. In addition, their results confirmed both an accumulation of isoproterenol-induced cyclic adenosine monophosphate and increased expression of RANKL. The dexamethasone treatment appeared to promote the general responsiveness of the osteoblasts to adrenergic stimulation, suggesting that glucocorticoid-induced bone loss may be mediated by alterations in the tonic state of sympathetic signal receptivity, favoring bone resorption.
Lifestyle Factors
Nutrition
Calcium and vitamin D
Calcium and vitamin D play a critical role in skeletal development and continuing bone health. Calcium is required for the maintenance of bone health. The amount of calcium required to meet the needs of the body changes throughout childhood and into adulthood, with peak nutritional needs occurring during adolescence. During periods of slower growth, the relationship between urinary calcium excretion and calcium intake is more pronounced than during the period of rapid growth in adolescence when calcium need is high.
Vitamin D nutrition
Vitamin D is essential for facilitating calcium absorption. Calcium regulation and the 25-hydroxyvitamin D–PTH axis is well established, and is illustrated by the inverse relationship between serum 25-hydroxyvitamin D and serum PTH. Severe vitamin D deficiency causes rickets or osteomalacia whereby new bone is poorly mineralized, causing bone softening and deformity. Less severe vitamin D deficiency often results in increased serum PTH leading to bone resorption, osteoporosis, and increased risk of fracture. The NHANES study compared the risk of hip fractures in adults for several ranges of serum 25-hydroxyvitamin D levels: below 16 ng/mL, risk of hip fracture was 60% higher; between 16 and 20 ng/mL it was 45% higher; between 20 and 25 ng/mL fracture risk was 36% higher; and between 25 and 30 ng/mL there was a nonsignificant increased risk of hip fracture of 13%. Two meta-analyses, published within the past 5 years, evaluated the effect of both vitamin D and calcium supplementation on bone health and fracture risk. These studies included a total of 114,625 adults from vitamin D–insufficient regions. The analyses concluded that vitamin D alone was not effective in reducing fracture rate (hazard ratio, 1.01; 95% confidence interval, 0.92–1.12); however, vitamin D intake of at least 800 IU/d combined with a calcium intake of 1000 to 1200 mg/d was effective for fracture prevention.
Sources of vitamin D include direct skin exposure to sunlight, few foods, and dietary supplements. Skin exposure to ultraviolet B radiation from the sun provides the predominant source of vitamin D. An individual in a bathing suit generates 10,000 to 25,000 IU of vitamin D2 after 1 minimal erythemal dose, which is the safest amount of radiation sufficient to produce redness in the skin. After hydroxylation in the liver and kidney to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, respectively, the active metabolite binds to the vitamin D receptor in a cell, and induces transcription of a responsive gene. Calcium-binding protein is a product of vitamin D–induced transcription, and mediates calcium transport across the intestinal mucosa.
The vitamin D receptor has been found in many tissues including bone and muscle cells, suggesting wide physiologic influence. The half-life of 25-hydroxyvitamin D in the body is approximately 15 to 20 days. In 2011, the Institute of Medicine (IOM) published recommendations, derived from a thorough review of the current literature, for the daily intake of vitamin D and calcium for children and adults. The IOM increased the 2008 American Academy of Pediatrics recommendations for vitamin D from 400 IU/d to 600 IU/d for children and adults 1 to 70 years old, and 800 IU/d for seniors older than 70 years. In a prospective trial assessing treatment response, 400 IU/d of vitamin D increased the serum 25-hydroxyvitamin D level in postmenopausal women by an average of 32.5 nmol/L over a 12-month treatment period. This increase was higher than previous predictions suggesting 400 IU/d would only raise serum 25-hydroxyvitamin D by approximately 10 nmol/L. With standard-dose supplementation, serum 25-hydroxyvitamin D is likely to plateau after 3 to 4 months. Therefore, when monitoring a patient’s response to supplementation, a serum 25-hydroxyvitamin D should be measured no sooner than 3 months after treatment begins.
The serum concentration of 25-hydroxyvitamin D 3 is considered the best available biomarker to measure the nutritional status of vitamin D. There are multiple assays in use, and clinicians should be aware that studies comparing interassay and interlaboratory testing of serum concentrations of 25-hydroxyvitamin D 3 have revealed significant variability in the results, making the assessment of vitamin D status in patients and the interpretation of vitamin D efficacy studies inherently more difficult.
Vitamin K
In addition, there is evidence in human intervention studies that vitamins D and K work synergistically toward improving bone density. Vitamin K is required for the γ-carboxylation of osteocalcin. In a recently published study evaluating the combined use of supplemental calcium, vitamin D, and Vitamin K on bone health, greater increases were observed in bone density within the lumbar spine of treated subjects when compared with the control group, who were treated with calcium and vitamin D.
Magnesium
Magnesium is the second most abundant intracellular cation, playing an important role in enzyme function and transmembrane ion transport. Magnesium deficiency has been associated with osteoporosis. Rates of magnesium deprivation sufficient to induce osteoporosis in animal studies are thought to occur commonly in the Western diet. Magnesium deficiency has been shown to increase substance P, TNF-α, IL-1β, and RANKL, with a decrease in OPG, favoring increased bone resorption.
Researchers have begun to evaluate multinutrient therapies for the treatment of osteoporosis. Genuis and Bouchard recently published findings from a series of patients who had failed bisphosphonate therapy and were treated using a combination of micronutrients chosen from the literature for their bone health properties. The treatment included 12-month supplementation with vitamin D 3 , vitamin K, strontium, magnesium, and docosahexaenoic acid. Serial bone densitometry was performed and demonstrated improved BMD in compliant patients. It was concluded that the supplementation regimen appeared to be at least as effective as bisphosphonates in raising BMD levels in the hip, spine, and femoral neck. No fractures occurred during follow-up in the micronutrient treatment group.
Physical activity
Weight-bearing physical activity is considered an intervention strategy for promoting optimal bone density in youth and to reduce bone loss in adults. Dynamic loading promotes greater bone-tissue gains than static loading, even if static loads produce large forces. Athletes involved in high-impact sports such as gymnastics show greater bone density than those involved in low-impact sports such as swimming. Health problems that reduce bone stimulation from mechanical loading result in bone loss, as illustrated by disuse osteoporosis caused by prolonged bed rest and immobilization. Reduction of mechanical stress on bone inhibits osteoblast-mediated bone formation and accelerates osteoclast-mediated bone resorption. Rittweger and colleagues performed a 35-day bed-rest investigation and assessed BMD 2 weeks after the initiation of bed rest, and reported reduction of bone mass in the cancellous bone–rich areas of 1% at the distal femur, 3% at the patella, and 2% at the distal tibia, with no changes seen at the distal radius. Results of a cross-sectional study completed by Garland and colleagues demonstrated more than 20% bone loss at the distal femur 3 months after injury in posttraumatic paraplegic and quadriplegic patients with spinal cord injuries. It is intriguing that high-frequency, low-intensity whole-body vibration has demonstrated bone-improving effects similar to those of mechanical force on bone density in both animal and human studies.
Fracture risk assessment
Bone quality may be evaluated in several different ways. Dual-energy x-ray absorptiometry (DXA), quantitative computed tomography (QTC), and bone turnover markers (BTMs) are some examples. QTC is a 3-dimensional nonprojectional technique used to quantify BMD in the spine, proximal femur, forearm, and tibia. There are several advantages of QTC in comparison with other densitometric techniques: cortical and trabecular bone can be separated, trabecular volumes of interest are largely independent of degenerative changes in the spine, and 3-dimensional geometric parameters can be determined. BMD, as measured by QTC, is a true density, measured in g/cm 3 , in contrast to DXA, which determines an areal density measured in g/cm 2 . However, QTC has not become the standard measure in the clinical setting, where DXA scanning remains the technique of choice for the assessment of pathologic bone conditions. DXA provides reference data from infancy to postpuberty, taking into account effects of age, sex, race, maturation, and size on BMD and bone mineral content (BMC), and allows a determination of the degree of departure from normative values in the form of t- and z -scores. BMD is the standard for evaluating fracture risk and is easily measured in patients. However, DXA provides only an estimate of BMC, and derives the BMD by dividing the BMC by the projected area of bone evaluated. The derived BMD is not a measure of volumetric density, providing no information about the depth of bone. In addition, bones of larger width and height also tend to be thicker, and bone thickness is not factored into DXA estimates of BMD, resulting in underestimates for short individuals. Children, particularly those with smaller bones, may appear to have a mineralization disorder. This effect is clearly important when assessing children’s bone health with DXA. In 2011, revised pediatric DXA reference curves were published by Zemel and colleagues derived from their data on 2014 healthy children. These investigators recommended adjusting for height in children, particularly those whose height is at the extremes of the normal growth continuum, and included parameters and an equation to adjust for these differences. However, the revised reference curves have yet to be evaluated as a predictor of fracture risk. Previously reported studies have shown a weak inverse relationship between BMD as determined by DXA and subsequent fracture risk.
Measures of bone turnover
BTMs are readily detectable peptides released from the bone matrix and through collagen degradation; however, their variability is of practical concern. The release of these substances may reflect bone turnover and indicate abnormalities in bone and mineral metabolism; however, marker concentration does not necessarily correlate with the severity of the mineralization process. Commonly used biomarkers of bone turnover include serum osteocalcin, amino-terminal propeptide of type I procollagen, and urine and serum β-isomerized C-telopeptides. Newer BTMs include P1NP and TRACP5b. P1NP is a marker of early osteoblast proliferation, and TRACP5b is a marker of osteoclastic activity and bone resorption. It is the only form of TRACP enzyme secreted by osteoclasts.
Bone health in selected neuromuscular diseases
Duchenne Muscular Dystrophy
Bone health has been studied more extensively in DMD than in any other NMD, and reports date back to 1941. DMD is an X-linked recessive disorder characterized by progressive muscle weakness, leading to premature death. DMD affects about 1 in 3600 to 6000 males and is the most common form of muscular dystrophy. The DMD phenotype is caused by a mutation in the dystrophin gene, resulting in the translation of a defective dystrophin protein that is rapidly degraded. This process results in a severe reduction or absence of dystrophin protein within muscle and destabilizing effects on the sarcolemmal membrane.
DMD is typically first recognized in affected boys by 5 years of age. Early signs include calf pseudohypertrophy and proximal leg weakness, which impairs mobility and results in the classic Gower maneuver observed when an affected boy transfers from the floor to standing. By definition, boys diagnosed with a DMD phenotype lose independent ambulation before age 16 years, with the most typical time for transition to wheelchair occurring before the earlier teens. DMD is a multisystem disorder including progressive respiratory and cardiac dysfunction, which are often the sequelae responsible for reduced life expectancy.
Decreased BMD and fractures occur commonly in DMD, and have been reported repeatedly. The recent shift in consensus recommendations to support routine use of corticosteroids for disease-modifying treatment, aimed at prolonging ambulation in DMD patients, has heightened concern for bone health because of the known negative impact of chronic glucocorticoid treatment in other patient populations. No rigorous studies have been published examining the effects of corticosteroids on bone health in DMD; however, since 2004 a growing number of international workshops have convened to address this issue.
Limitations in the current literature addressing bone health in DMD include lack of concurrent age- and sex-matched healthy controls, and variations in study design including methods and outcome measures, which make it difficult to reliably interpret the results reported for many of the biochemical indicators of bone health.
Scoliosis in DMD
Scoliosis has been reported to occur in up to 90% of patients with DMD. It is one of the most obvious observations that dystrophin deficiency has accompanying effects on bone health and development. However, a retrospective study of 143 patients diagnosed with DMD and comparing steroid-treated with steroid-naïve boys revealed an increase in the mean age at transition to wheelchair by approximately 3 years and reduced scoliosis severity, limiting the need for surgical stabilization in the treated group. The mean degree of scoliosis measured in the nontreated group was 33.15° ± 29.98° versus 11.58° ± 15.65° for the treated group ( P <.0001).
Fractures in DMD
Estimates suggest that up to 25% of boys with DMD will experience a long bone fracture with subsequent loss of ambulation. In a large retrospective study that examined the case reports of 378 boys with DMD, 20.9% or 79 patients had experienced fractures. Falls were the most commonly reported cause of fracture. Of patients with fractures, 48% were between 8 and 11 years old, and 48% were ambulatory. In boys ambulating with the assistance of knee-ankle-foot orthoses, upper limb fractures occurred most commonly (65%). Lower limb fractures were most prevalent in independently mobile and wheelchair-dependent patients (54% and 68%, respectively). Of independently ambulant patients and those using orthoses, 20% and 27%, respectively, lost mobility permanently as a result of the fracture. The investigators reported the fracture prevalence of those exposed to corticosteroids as similar to that of the unexposed group. However, they did not examine steroid regimens or ascertain the interval between corticosteroid initiation and fracture in the study population.
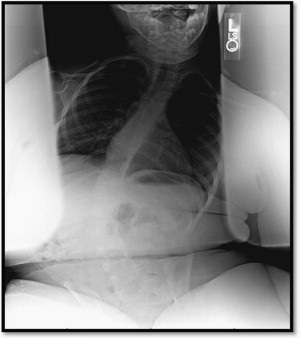
Before the broad initiation of corticosteroid treatment in DMD, reports of vertebral fractures were relatively rare. However, recent retrospective studies have reported increased vertebral fracture rates in patients treated with corticosteroids in comparison with steroid-naïve patients ( Fig. 1 ). Of the 143 patients studied by King and colleagues, 75 patients had received steroid treatment of at least 1 year’s duration, and 68 were steroid naïve. No vertebral fractures were identified in the nontreated group, but 32% of the treated group suffered a vertebral fracture. Another study of 79 DMD patients by Houde and colleagues, including 37 patients treated with deflazacort, reported similar frequencies of limb fractures between deflazacort-treated (24%) and untreated (26%) boys. Vertebral fractures, however, occurred exclusively in the treated group (7 of 37 patients).
In a study of 25 DMD patients who were treated with daily corticosteroids for a median duration of 4.5 years, 40% sustained vertebral fractures. Eight were identified due to symptomatic backache, and 2 had fractures detected on spinal radiographs taken because of low BMD. The first reported fracture occurred at 40 months into treatment. These reports highlight the importance of including fracture surveillance as part of regular follow-up care for the steroid-treated DMD population (see Table 1 ).
BMD in DMD
Many DMD studies have reported decreases in BMD. Aparicio and colleagues evaluated the BMD of 10 DMD boys by DXA. None of the boys had been treated with corticosteroids and all were community ambulators between the ages of 6 and 11 years. Eight of the 10 boys had osteoporosis of the proximal femur, and the remaining 2 had abnormal measures more consistent with osteopenia. Two of the 10 patients had decreased BMD of the spine within the osteoporotic range, and 3 were osteopenic. The study did not include aged-matched controls for comparison. In a prospective study where 30 steroid-naïve DMD boys underwent from 2 to 17 DXA evaluations over a 7-year period, Larson and Henderson evaluated BMD through the time spanning independent ambulation to wheelchair dependence. While ambulatory, the BMD in the lumbar spine was only slightly decreased (mean z -score, −0.8); however, significant decreases occurred in BMD with loss of ambulation (mean z -score, −1.7). By contrast, BMD of the proximal femur was diminished before the loss of ambulation (mean z -score, −1.6), and progressively worsened to nearly 4 standard deviations below age-matched nondiseased controls (mean z -score, −3.9). A study by Söderpalm and colleagues assessed the bone health of 24 boys treated with glucocorticoids in comparison with controls. BMD differed significantly from controls at all ages, and these differences in values between patients and controls increased significantly with age. While the above studies confirm baseline BMD abnormalities in both nonsteroid and steroid-treated DMD patients, the question still remains: do corticosteroids improve baseline BMD by improving muscle strength and prolonging ambulation, or do they have additive bone-deteriorating effects worsening the bone health of DMD boys?
The recent study by Rufo and colleagues, aimed at exploring the mechanism responsible for the deterioration of bone health in DMD, is one of the most comprehensive studies of bone health in DMD to date. The study design incorporated multiple outcome measurements across human subjects, the mdx mouse model, and in vitro culture of osteoclast precursors and primary osteoblasts. Corticosteroid-naïve DMD patients were compared with aged-matched controls and mdx mice were compared with wild-type mice. Differences observed in BMD between the mdx and wild-type mice were consistent with differences in BMD between DMD and control subjects. The investigators also identified increased populations of osteoclasts, RANKL/OPG ratio abnormalities that favored bone resorption, and significantly increased levels of IL-6, a recognized inhibitor of osteoblast function.
Vitamin D status in DMD
Because of the challenges previously described, vitamin D and calcium nutritional status are very difficult to analyze because quality data on normal vitamin D and calcium status in healthy children is limited. Nonetheless, it is clear that many boys with DMD are insufficient or deficient in vitamin D. Bianchi and colleagues published a follow-up study of 33 children with DMD being treated with a fixed dose of prednisone (1.25 mg/kg every 2 days). Patients were observed for the first year and then treated with vitamin D 3 (0.8 μg/kg per day) plus adjustment of dietary calcium to the internationally recommended daily allowance for 2 additional years. During the observation year, BMC and BMD decreased in all patients. At the end of the 2-year supplementation phase, BMC and BMD significantly increased in more than 65% of patients. Bone metabolism parameters and BTMs were also reported to have normalized in most patients (78.8%). These results reveal important aspects about the bone disorder in DMD patients. Components of osteomalacia are suggested by observed improvement of low BMC and BMD with repletion of vitamin D. However, aspects of osteoporosis remain even after improved vitamin D and calcium nutritional status.
Amyotrophic Lateral Sclerosis
ALS is a rapidly progressive neurodegenerative disease caused by the loss of both upper and lower motor neurons throughout the neuraxis, including the motor cortex, brainstem, and spinal cord. The loss of motor neurons most typically results in a mixed picture of spasticity, diffuse muscular atrophy, and weakness. Most cases of ALS are presumably acquired and occur sporadically, with only about 10% occurring by familial inheritance. The etiology of sporadic ALS is as yet unknown, but data suggests a multifactorial process ending in a final common pathway of motor-neuron apoptosis. Familial and sporadic ALS cases are clinically indistinguishable.
ALS most commonly strikes individuals between the ages of 50 and 74 years, with a reported mean age of onset reported to extend from 58 to 63 years. The incidence is approximately 1 to 3 per 100,000 with an overall prevalence rate of 5 to 10 per 100,000, making it one of the most common NMDs worldwide. The 50% median survival rate is approximately 2 years after diagnosis. Abnormalities in calcium metabolism have long been identified in ALS patients. In a retrospective study published in 1976, the investigators reviewed the records of 39 patients to discover that 20% had abnormal serum calcium levels and more than 50% showed radiographic evidence of bone abnormalities. However, the literature regarding bone health in ALS remains very limited.
Fractures in ALS
Fractures occur commonly in ALS. Disease progression causes early discoordination and imbalance, which promotes an increased frequency of falls in patients as they approach the time of loss of independent mobility. In fact, in a multicenter clinical trial, falls were the third most common adverse event reported, and fall-related deaths have been reported to occur in 1.7% of ALS patients. Several studies have found that fractures are more frequent among ALS patients than in controls. In the case-control study by Campbell, ALS patients had 14% more fractures than controls. In a retrospective study, Kurtzke and Beebe reviewed the military records of 504 men who died of ALS and found excess hospital admissions for trauma and fracture, particularly of the limbs and skull. The increased rate of fractures, skeletal abnormalities, and trauma in ALS patients was initially thought to be a risk factor for developing disease; however, further population studies investigating an association between trauma and ALS have not established a causal link, suggesting that these findings are more likely due to early prediagnostic symptoms of disease.
In an attempt to lower the incidence of fractures in their ALS population, Sato and colleagues treated 82 ALS patients, after random assignment, to daily treatment with 400 mg of etidronate or placebo over a 2-year period. At baseline, both groups had low BMD with high levels of serum ionized calcium and BTMs. In the etidronate group, serum calcium and marker levels decreased significantly during the study period, whereas the levels in the placebo group were increased. BMD decreased in all patients but was substantially slowed in the etidronate group compared with placebo (3.6% vs 12.1%; P <.0001). Fractures occurred in 7 patients in the placebo group and 1 patient in the risedronate group, with relative risk in the risedronate group in comparison with the placebo group of 0.14 (95% confidence interval, 0.02–1.11). These data suggest that there may be an opportunity to initiate early treatment directed at bone health, and perhaps avoid the morbidity associated with poor bone health and abnormal BMD in ALS.
BMD and vitamin D status in ALS
Few studies have evaluated bone density and markers of bone health in ALS. In addition to reducing fracture-related morbidity, there is an opportunity to examine the effects of an asymmetric progressive neurodegenerative disease on bone health in this unique patient population. With mounting evidence supporting a major role of the nervous system in bone metabolism, further studies may add to our understanding of the basic biological mechanisms involved in the maintenance of healthy bone. One small study examined the effects of chronic asymmetric neurologic impairment on bone density, evaluating patients with cerebrovascular disease (CVD), Parkinson disease (PD), and ALS. A high incidence of osteoporosis and right/left difference in osteopenia was reported. CVD and PD patients with asymmetric osteopenia showed an association between clinical symptoms, peripheral circulatory symptoms, and predominant osteopenia. Although the muscle strength of PD patients was reported as normal, the more severely affected side for PD symptoms and autonomic symptoms coincided with predominant osteopenia in the body. Increased bone resorption was detected in all ALS patients.
In an earlier study, preceding their etidronate clinical trial, Sato and colleagues assessed the bone health of 11 patients with ALS using bone density and serum biochemical indices of bone metabolism in a comparison with controls. The investigators identified vitamin D deficiency in 2 and insufficiency in 9 ALS patients. In addition, the mean serum 25-hydroxyvitamin D was significantly lower in ALS patients than in controls (14.0 ± 3.7 ng/mL vs 25.2 ± 4.0 ng/mL). Serum PTH and ionized calcium were elevated in 8 and 6 patients, respectively. z -Scores of metacarpal bone density were in the deficient range for 7 of the 11 ALS patients. These data underscore the potential importance of hypovitaminosis D and compensatory hyperparathyroidism in the development of osteopenia in patients with ALS.
In addition, as the general population ages and the ALS community identifies disease-slowing treatments, increasing the prevalence of patients with ALS, poor bone health will likely become a more frequent and thus a more costly problem, owing to fracture-related morbidity. Further studies are urgently needed to elucidate the prevalence of metabolic bone disease in ALS, determine the best diagnostic and treatment strategies, and evaluate the efficacy and timing of interventions. The potential to evaluate the contributions of nutritional (vitamin D), endocrine (parathyroid), and neurologic impairment and bone health–directed treatment in ALS, with its asymmetric, progressive disease course, is intriguing. Because of the complex nature of bone metabolism and maintenance, the implications of these studies may provide widespread benefits for patients with diverse causes of neurologic and neuromuscular disease, thus contributing to a better understanding of the basic pathology of bone disease.
Spinal Muscular Atrophy
There are several clinical presentations of SMA, all of which involve selective destruction of anterior horn cells. The various subtypes of SMA are clinically heterogeneous, with some rare forms affecting distal or bulbar muscles only. However, SMA usually has onset of symptoms in childhood and is inherited as an autosomal recessive trait. The incidence of SMA is about 1 in 10,000 live births with a carrier frequency of 1 in 50.
The gene responsible for childhood-onset SMA has been mapped to chromosome 5q11.2-13.3. The causative gene, survival motor neuron 1 (SMN1), and a disease-modifying gene, survival motor neuron 2 (SMN2), have been identified. The most common abnormality of the SMN1 gene is a deletion of exon 7, but other exon deletions and point mutations can be disease causative. The SMN protein is ubiquitously expressed in all tissues, with high levels in the nervous system. Recent data indicate that SMN1 deficiency alters stoichiometry of small nuclear ribonucleoproteins and leads to splicing defects for numerous genes in all cells, including motor neurons.
The full-length transcripts of SMN1 and SMN2 encode proteins with an identical sequence; however, structural differences in the SMN2 gene cause frequent but not absolute exclusion of exon 7 during splicing. The copy number of SMN2 varies in the population, and this variation appears to have important disease-modifying effects on SMA severity, with more SMN2 gene copies resulting in a less severe disease.
The most common forms of SMA are often referred to as types I, II, and III. SMA I is a severe disorder often resulting in death before 2 years of age, although longevity has been increased as a result of better medical management of disease sequelae. Children with SMA I never attain the ability to sit independently. SMA II is less severe, with signs and symptoms becoming apparent in the first 18 months of life. These children sit independently but do not ambulate without assistance. SMA III has later onset, and all early developmental milestones including independent ambulation are acquired. In prior studies looking at SMA II and III over a 10-year period, SMA II subjects showed marked weakness and progressive decline of strength whereas SMA III subjects had less weakness and a relatively static, slowly progressive course. SMA III is consistent with a normal life span.
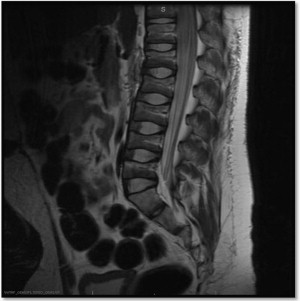
Scoliosis in SMA
Similar to DMD, an early sign of poor bone health is the high prevalence of severe progressive scoliosis in SMA. Scoliosis, with increasing pelvic obliquity, is a common feature occurring in the early childhood of patients with SMA II ( Fig. 2 ). Several studies have been published documenting the incidence, severity, and progression of scoliosis, while comparing phenotypes and treatment outcomes. Rodillo and colleagues reviewed the incidence and severity of scoliosis in 37 patients with SMA II and 26 with SMA III. In SMA II, scoliosis had an early onset and rapid progression before puberty. The rapid progression occurred despite consistent use of a spinal brace, and spinal fusion was needed in all cases. In patients with SMA III, scoliosis was more variable. Scoliosis was present in 30% of patients and progressed rapidly during puberty in those who lost ambulation. Progression of scoliosis was slow in all who maintained ambulation, even if ambulation was assisted by orthoses. Granata and colleagues reviewed 63 spinal radiographs of affected patients. All but one of the SMA II patients, and all of the SMA III patients who stopped ambulating had scoliosis, ranging from 10° to 165°. Of the 19 ambulatory SMA III patients 12 had scoliosis, ranging from 10° to 45°. Mean age at onset was 4 years 4 months in SMA II, and 9 years 10 months in SMA III. The severity of scoliosis in SMA has been reported to affect respiratory function, with a near linear inverse relationship to the patient’s percent predicted forced vital capacity. With surgical correction, improvement in respiratory outcome measures have been observed, suggesting the potential of improved respiratory function if treatment-altering bone health is efficacious and reduces scoliosis severity.