Fig. 10.1
It is clear to see why gymnasts may have a higher rate of spondylolysis
Furthermore, age is a risk factor for LBP. During adolescence, the spine is at risk of spondylolysis because growth cartilage is more susceptible to deforming forces than ligament or bone. Compressive forces on vertebrae can rupture cartilaginous end plates, causing Schmorl nodes to form, or the ring apophysis to produce limbus vertebrae. Conversely, tensile forces can result in vertebral body apophysitis or apophyseal avulsions. Also, as linear skeletal growth occurs, mineralization of bone is delayed and is more susceptible to fracture [23, 24]. Additionally, rapid growth during the adolescent growth spurt increases muscle-tendon tightness resulting in decreased flexibility and increased risk of injury.
History and Physical Exam
History
A careful history in young athletes with back pain will usually lead to a correct diagnosis. Duration, intensity, and location of symptoms should be elicited. The location of symptoms is important as a more localized area of pain is more suggestive of an identifiable cause as opposed to vague, diffuse back pain. Clinicians should determine which sports he/she plays, position of play, and volume of training, as certain sports have a significantly increased risk of back injury. Similarly, certain positions in sports, such as offensive linemen in football, have a higher incidence of spondylolysis [21]. Additionally, volume of training may suggest overtraining as a cause for back injury.
It is important to determine if pain is localized to the back or whether there are radicular symptoms. Radicular pain, numbness, or dysesthesias suggest nerve compression. It is also critical to inquire about cauda equina symptoms such as bowel or bladder dysfunction, saddle anesthesia, or severe bilateral lower extremity pain, which indicate a surgical emergency.
Onset of pain may also give clues to diagnosis. Pain associated with sports may occur during or after activity. Night pain may indicate a more serious condition and presence of weight loss, fevers, or generalized malaise should be determined. A nutritional history is critical, especially in female athletes in which there are concerns for the “female athlete triad,” as this population is more susceptible to stress fractures [25].
Physical Exam
A thorough physical exam is as important as the history in obtaining the correct diagnosis. Observation should always be the first part of the exam, particularly noting overall body habitus and posture. A pale, cachectic patient may suggest an underlying malignancy, infectious disease, or nutritional disorder. Coronal and sagittal alignment should be evaluated for trunk imbalance, scoliosis, lordosis, or kyphosis. Patients may present with a “sway-back” deformity indicating excessive lumbar lordosis and associated hip flexion contracture. Additionally, the examiner should look for cutaneous abnormalities such as café au lait spots, sacral dimples, hemangiomas, or hair patches.
Palpating the spine and paraspinal musculature can help delineate bony injury versus muscular strain. Tenderness along spinous processes may indicate fracture, ligamentous injury, or apophysitis. If the sacroiliac joints are tender, a flexion abduction external rotation (FABER) maneuver of the hips may help confirm sacroiliitis. Finally, the greater trochanters should be palpated laterally for tenderness, indicating bursitis or gluteus medius tendinitis, and in the posterior peritrochanteric region, indicating piriformis syndrome or other short external rotator inflammation.
Range of motion in flexion and extension should be assessed as well as whether pain is elicited during flexion or extension. Flexion pain is less specific in young athletes but can suggest disc pathology. A patient with a disc herniation may be apprehensive about lumbar flexion and the Valsalva maneuver [26]. Limited flexion may be secondary to pain or tight hamstrings. An Adams forward bend test should be performed to assess for a rib hump or asymmetry which suggests scoliosis. Although scoliosis is not typically painful, a left-sided thoracic curve may represent an underlying spinal cord abnormality. Extension pain is more specific for spondylolysis or posterior element pathology. Stork testing (single-leg hyperextension) can increase specificity and can localize laterality of the spinal abnormality.
A complete neurological exam is essential for any patient with LBP, particularly those with radicular or nerve-like complaints. This should include sensory and motor exam of nerve roots L2 to S1 [27]. A good start to the motor exam is to ask the patient to heel walk and toe walk. If they can do this without difficulty it indicates gross motor integrity of L4 and S1, respectively. Reflexes and signs of upper motor neuron irritability (i.e., clonus, Babinski testing, abdominal reflexes) should be tested. Nerve tension tests including straight leg raise, contralateral straight leg raise, Lasegue sign, and prone femoral stretch should be performed to assess for nerve root irritation.
A lower extremity exam may indicate contributing factors to LBP. In particular, an increased popliteal angle, indicative of tight hamstrings, can cause LBP but can also result from spondylolisthesis. A positive Thomas test, indicating hip flexor contracture, may be present in those with excessive lumbar lordosis.
Acute Injuries
Fractures
Pediatric thoracolumbar trauma accounts for approximately 0.6–0.9 % of all spine fractures, with sports accounting for 21–53 % of these [28–30]. Athletes involved in sports with axial loading, such as diving and snowboarding, and contact sports such as hockey and wrestling, have an especially high risk for fractures.
Patients with suspected thoracolumbar fracture should be managed according to advanced trauma life support (ATLS) protocol. Proper spine boarding technique should always be performed to prevent additional injury. A careful exam including palpation for bony tenderness or step-off, as well as a detailed neurologic exam, is critical.
Plain radiographs, historically, have been the first line in imaging. However, computed tomography (CT) has become quicker and easier, frequently becoming the initial imaging choice in adults. In pediatric patients, however, radiation exposure must be considered, as a single CT scan results in a theoretic 13–25 % median excess relative risk of thyroid cancer induction [31]. When interpreting spinal X-rays, Denis’ three column theory can aid in description of the injury and stability of the fracture [32]. Although a two-column injury typically indicates instability, there are two column fractures that are stable (i.e., stable burst fractures). If neurologic findings are present, magnetic resonance imaging (MRI) should be obtained to evaluate for herniated discs, hematoma, neuroforaminal encroachment, ligamentous injury, or spinal cord edema.
Younger children (<9 years old) may have a higher incidence of neurological injury without skeletal abnormality, known as “spinal cord injury without radiographic abnormality” (SCIWORA). This occurs because the immature spine is more elastic, allowing for greater ranges of motion and displacement without fracture. SCIWORA occurs in 30–40 % of spinal cord injuries in pediatric patients [33, 34] and 23 % of SCIWORA patients may have a delayed presentation of neurologic injury ranging from 6 to 72 h after injury [35].
Most pediatric thoracolumbar fractures are stable and do not result in neurological injury or long-term problems. Spinous process and transverse process fractures account for 23 % of all spine fractures in young athletes, while compression fractures represent 48 %. Isolated spinous process and transverse process fractures usually result from blunt trauma and can be managed with pain control and return to activities as tolerated. Compression fractures can be treated with activity modification, a thoracolumbarsacral orthosis (TLSO) for 6–8 weeks and gradual return to sports.
Burst fractures occur from an axial load injury and are classified as stable or unstable. Stability of burst fractures is controversial, but in contrast to adults, the percentage of canal compromise does not necessarily correlate with the risk of neurologic injury. This may be because the immature spine has a larger canal diameter with respect to the spinal cord [36, 37]. Stable burst fractures can be managed in a hyperextension cast or TLSO brace for 8–12 weeks. Unstable fractures are treated with posterior pedicle screw implantation with or without arthrodesis and with or without decompression.
Apophyseal Fractures and Herniations
Apophyseal ring fractures occur in children and adolescents aged 10–14 years and result from a separation of the vertebral apophysis from the spongiosa layer of the vertebral body. This injury, seen almost exclusively in the skeletally immature, is analogous to a herniated disc in adults. The apophysis can herniate into the spinal canal or into the neural foramen causing nerve root impingement. The apophysis may spontaneously reduce, however, and X-rays may appear normal. If X-rays are carefully scrutinized, a small bony fleck may be seen posterior to the vertebral body. MRI is important to determine how much injury has occurred. Adolescent athletes involved in weight lifting or gymnastics are at increased risk. The classic presentation is an adolescent with radicular pain after weight lifting. Ninety percent of apophyseal ring fractures occur at the L4–5 level.
Treatment consists of anti-inflammatories, activity modification, and 8 weeks in a TLSO, if there are no significant neurologic findings or very mild radicular symptoms. Surgical decompression to remove the limbus is warranted if neurologic deficits are present.
Acute Disc Herniation
Discogenic causes for back pain, including disc herniation, account for 11 % of LBP in young athletes [4]. The majority of disc herniation occurs after 12 years of age and 92 % occur at the L4–5 or L5–S1 levels. Athletes involved in collision sports or weight lifting are at increased risk. Between 30 and 70 % of adolescents with acute disc herniation have vertebral anomalies such as scoliosis, transitional defects (lumbarization and sacralization), schisis, and canal narrowing [38]. Genetic and familial factors may contribute to early disc disease [39, 40]. Finally, there is increased incidence of acute disc herniations in patients with growth cartilage abnormalities of the lumbar spine, such as Schmorl nodes and Scheuermann’s disease.
Patients with a herniated disc may have apprehension with lumbar spine flexion or Valsalva maneuver. A scoliotic posture may be assumed as a compensatory mechanism to relieve pressure off a compressed nerve root. Additionally, straight leg raise will be positive two-thirds of the time [41].
First-line treatment of an acute herniated disc is nonsurgical, including rest, anti-inflammatories, and physical therapy with gradual return to activities. More aggressive non-operative treatment could include a rigid brace and epidural steroid injections. Unfortunately, conservative therapy is less effective in adolescents when compared to adults [42, 43]. One study of surgically treated lumbar disc herniations in children and adolescents revealed that as few as 40 % of adolescents with herniated lumbar discs responded to conservative therapy and recurrence was common [44].
Sprains, Strains, and Contusions
Although common, muscular strains, ligamentous sprains, and contusions are diagnoses of exclusion. Ligamentous sprains and muscular strains account for 20 % of back pain in adolescent athletes [4]. Injury to the interspinous ligament is the most common sprain [45]. A contusion occurs after blunt trauma to soft tissues and may cause hematoma formation.
Sprains, strains, and contusions cause acute pain in the first 24–48 h and are often associated with spasms and localized, palpable tenderness over the affected area. Imaging will be negative except MRI, which will show localized edema within the soft tissue area of injury. Recurrences can be common and may become chronic. Acute management includes rest, ice, and anti-inflammatories. Physical therapy should target core muscular imbalances, core strengthening, and hamstring stretching. Modalities including electrical stimulation, massage, and ultrasound may provide some benefit. Gradual return to sports occurs as symptoms resolve.
Chronic/Overuse Injuries
Spondylolysis and Spondylolisthesis
Spondylolysis is an anatomic defect of the pars interarticularis without displacement of the vertebral body. It usually results from a chronic cyclic loading of the inferior articular facet onto the pars interarticularis of the inferior vertebrae during repetitive hyperextension [46]. The most common vertebrae involved is L5 and the defect may be unilateral or bilateral. Patients with L4 spondylolysis are more frequently symptomatic [47].
Spondylolisthesis refers to the translation of one vertebra relative to the adjacent caudal vertebral segment. The most common location for spondylolisthesis in children and adolescents is at L5–S1. Dysplastic spondylolisthesis is more likely to progress (32 %) compared to the isthmic type (4 %) and is more likely to need surgery [48–50]. Although many patients with isthmic spondylolysis present with some degree of spondylolisthesis, <4 % of children and adolescents show slip progression in adulthood [48, 51]. Patients diagnosed before the adolescent growth spurt, females, and slips >50 % have a higher likelihood of progression [52].
The prevalence of spondylolysis is age dependent. A prospective study of 500 children followed from first grade for 45 years found a prevalence of spondylolysis of 4.4 % among 6-year-olds and 6 % in adults [53]. Although relatively uncommon in the general population, spondylolysis is more prevalent in athletes due to repetitive forces on the back.
Spondylolysis is the most common cause for back pain in young athletes, comprising 47 % of back pain in this population [4]. Once believed to be more common in boys, recent studies have shown equal prevalence [25]. Certain female-dominated sports have an increased risk, including gymnastics, ballet, and figure skating [9, 12–16]. Spondylolisthesis, however, is more common in females [11, 16, 17, 19].
There may be a genetic predisposition in developing spondylolysis. In family studies of patients with isthmic spondylolysis and spondylolisthesis, 22–26 % of first-degree relatives had similar radiographic changes, but most were asymptomatic [54, 55]. Children of European descent have two to three times the risk of developing spondylolysis and spondylolisthesis compared with those of African descent [53].
Back pain associated with spondylolysis becomes worse with activity; hyperextension with rotation is particularly painful. Physical exam typically reveals hamstring tightness and pain with “stork” testing. Patients with slips may have paresthesia, neurologic deficit (particularly L5), and positive tension signs.
Initial imaging for spondylolysis and spondylolisthesis include standing posterioanterior (PA) and lateral radiographs. Traditionally, supine oblique radiographs would be included to show the classic “scotty-dog” sign. However, obliques are only 32 % sensitive for spondylolysis while doubling the radiation exposure [56]. Additionally, there is no increase in sensitivity or specificity in detecting spondylolysis when comparing two-view versus four-view radiographs [57]. Lateral images are important for detecting a pars defect and documenting the degree of spondylolisthesis [58]. Slip angle can also be measured on the lateral radiograph. A slip angle >50° is associated with greater risk of progression, instability, and postoperative pseudoarthrosis [52]. Additionally, pelvic incidence (PI) can be measured on lateral X-rays (Fig. 10.2). Recent studies have shown a direct linear relationship between PI and severity of spondylolisthesis, suggesting that pelvic anatomy may directly influence the development of isthmic spondylolisthesis. PI was significantly higher in patients with low- and high-grade isthmic spondylolisthesis compared with controls [59]. Increased PI results in increased lordotic stress on the lumbar spine.
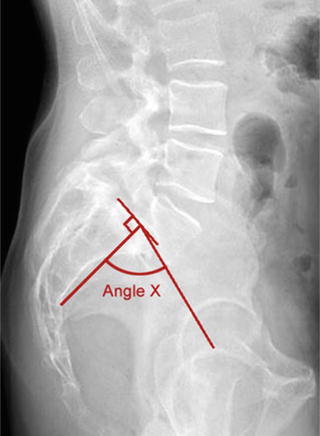

Fig. 10.2
When performing measurements on spinopelvic radiographs, pelvic incidence (PI) most closely correlates with the isthmic spondylolisthesis grade. It is measured by taking the angle subtended by an initial line from the center of the femoral head to the midpoint of the sacral end plate and a second line perpendicular to the center of the sacral endplate. PI is relatively constant during childhood (~47°), increases during adolescence, and remains constant in adulthood (~57°). Unlike many other parameters of pelvic morphology, PI is not affected by changes in posture. A low PI indicates low shear forces at the lumbosacral junction and less lumbar lordosis (reprinted from Hanson et al. [59]; with permission)
When radiographs are normal but clinical suspicion is high, single-photon emission computed tomography (SPECT) is the most sensitive method for detecting spondylolysis [60]. SPECT may also show osseous healing potential as increased signal uptake correlates with metabolically active bone [61]. Additionally, decrease in tracer uptake on serial SPECT scans has been correlated with improvement in signs and symptoms [46]. MRI can also be used when radiographs are normal but suspicion is high. In addition to avoiding radiation, MRI can detect bone marrow edema suggestive of a “pre-spondylolysis” [62]. Detection of “stress reaction” in the pars may increase the rate of bony union because early treatment can prevent frank fracture. One study comparing MRI and CT in detection of spondylolysis found that MRI was 92 % sensitive in detection of pars defect and found 11 lesions in 9 patients that had negative CT scan [63]. However, MRI for evaluation of back pain has a high false positive rate and positive predictive value [64]. One study showed that MRI detected pars abnormalities in 6 of 22 asymptomatic elite rowers [65]. Another study in young asymptomatic elite tennis players showed that only 4 % had no MRI abnormality [66].
Once spondylolysis has been diagnosed, thin-cut CT, performed with a reverse gantry angle, is the best imaging modality to define bony anatomy (Fig. 10.3). It can reveal sclerosis of the pars and size of the gap in the pars defect, which may assist in determining healing potential. CT is the test of choice to follow healing of spondylolysis using serial imaging [67].
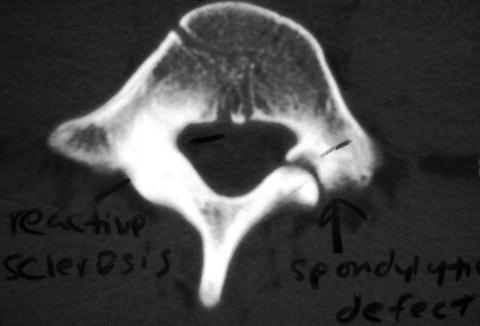

Fig. 10.3
CT scan is the imaging test of choice to define structural anatomy and follow serially to assess bony union
Initial management of spondylolysis is non-operative with activity modification and activity restriction for 3–6 months. Physical therapy is important to stretch hamstrings, strengthen core musculature, and perform specific anti-lordotic exercises, which have been shown to decrease pain and disability [68].
Although bracing is controversial, a lumbar brace, such as the modified Boston brace, has been shown to be superior to activity modification alone [11]. The brace is molded in 0°–15° of anti-lordotic flexion and worn for 24 h a day for the first 4–6 weeks, followed by weaning. Return to sports begins once there is painless extension and rotation of the lumbar spine. As the athlete returns to play, the brace is worn only during sports and is discontinued once the athlete has remained pain free for 3–4 months. This regimen has resulted in good to excellent results in 78 % of patients [69] with a 72–89 % rate of successful return to sports [51, 70]. Other studies have shown bony healing with use of a rigid brace, a soft brace, or no brace [71–74].
Resolution of symptoms does not necessarily indicate bony union of a pars defect. If pain resolves but thin-cut CT reveals a persistent pars defect then a fibrous union has occurred. Fibrous union frequently leads to a good clinical result [51, 61]. A meta-analysis showed only a 28 % rate of bony healing of spondylolytic defects despite an 84 % success rate in patients treated non-operatively; 71 % of unilateral defects healed while only 18 % of bilateral defects healed [75].
Most young athletes with spondylolysis or spondylolisthesis can be treated conservatively. Surgical treatment is reserved for progressive spondylolisthesis, neurologic deficit, or painful nonunion and persistent back pain [76]. If modification of sporting activities is unacceptable then pros and cons of surgery need to be thoroughly discussed. Patients should be reminded that long-term prognosis of spondylolysis without surgery is favorable and that continuing sports, although painful, will not necessarily worsen the spondylolysis. Many athletes may choose to tolerate some pain and continue sports; other athletes are unwilling to accept any activity limitations and would prefer surgical management [77].
Surgical options for spondylolysis are direct repair versus posterior lumbar fusion. Direct repair is performed at levels above L5, while for L5 itself, debridement of fibrous tissue and in situ fusion with autogenous iliac crest bone graft are the gold standard. Methods for achieving union include posterior wiring of the transverse process and spinous process, pedicle screw and hook techniques, or Buck translaminar interfragmentary screws. Results from Buck fusion are the most studied with a painless union rate of 88 % and return to sports of 82 % [78, 79]. Fusion is indicated if there is spondylolisthesis or a degenerative disc at L5–S1. Pedicle screw instrumentation with rods is the currently preferred method [80].
Lordotic Low Back Pain
Lordotic back pain is the second most common cause of LBP in young athletes [4]. During the adolescent growth spurt, the thoracolumbar fascia and interspinous ligaments may tighten and decrease flexibility, resulting in lordotic LBP. Pain may result from traction apophysitis or impingement of the spinous processes [4] (Fig. 10.4). Another possible cause of pain is excessive stress on the facet joints. In addition, Bertolotti syndrome, characterized by anomalous enlargement of the transverse processes of the most caudal vertebra, which may articulate or fuse with the sacrum or ilium and cause L4/5 disc disease, may also cause extension-based back pain [81].









