Chapter 147 Asthma
 General Considerations
General Considerations
Bronchial asthma is a hypersensitivity disorder characterized by bronchospasm, mucosal edema, and excessive excretion of a viscous mucus that can lead to ventilatory insufficiency. Asthma affects approximately 7% of the population of the United States and causes 4210 deaths per year. Although it occurs at all ages, it is most common in children below age 10. There is a 2:1 male:female ratio in children, which equalizes by the age of 30.1
Major factors involved in asthma are the following:
• Increased stress on the immune system due to factors such as greater chemical pollution in the air, water, insect allergens from mites and cockroaches, and food
• Earlier weaning and earlier introduction of solid foods to infants
• Higher incidence of obesity2
• Genetic manipulation of plants, resulting in food components with greater allergenic tendencies
In addition, multiple genetic variables may make certain individuals more susceptible to asthma. For example, researchers have demonstrated that a deficiency in the glutathione S-transferase M1, a gene involved in response to oxidative stress, may also make those with this deletion more susceptible to asthmatic attacks, thus supporting the need for antioxidant therapy in these individuals.3 The ADAM33 gene on chromosome 20p13 has been linked to the pathogenesis involved in airway remodeling (see mediators section later) and is probably a factor in corticosteroid resistance in some asthmatics.4 Other research teams have identified genes on chromosomes 7 and 12 as probable players in the pathogenesis of asthma.5,6
Major Categories
Asthma is often clinically classified according to the frequency of symptoms, forced expiratory volume in 1 second (FEV1), and peak expiratory flow rate.
Diagnostic Considerations
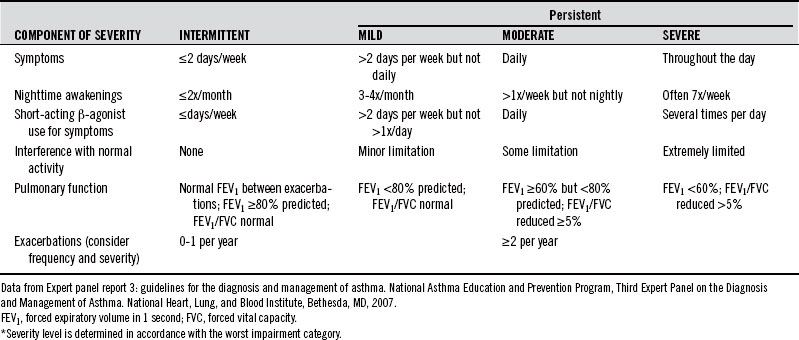
The U.S. National Asthma Education and Prevention Program (NAEPP) guidelines for the diagnosis and management of asthma (Table 147-1) state that a diagnosis of asthma begins by assessing whether any of the following list of indicators is present:
• Wheezing—high-pitched whistling sounds on expiration—especially in children. (Lack of wheezing and a normal chest examination do not exclude asthma.)
• History of any of the following:
• Symptoms occur or worsen in the presence of:
TABLE 147-1 National Asthma Education and Prevention Program (NEAPP) Classification of Asthma Severity Before Treatment in Adults and Youths 12 Years and Older*
Causes
Inflammation and Th1/Th2 Balance
Imbalances in T-helper cell immune responses appear to be a fundamental mechanism of immunologically mediated airway inflammation. CD4+ T-helper cells are generally categorized into Th1 and Th2 cells. Via the release of interferons and interleukin 2, the Th1 pathway is increased in the immunologic responses to cancer, multiple sclerosis, viruses, and type IV hypersensitivities. Th2 responses are related to increases in interleukins 4, 6, 9, and 13; IgE, eosinophilia; and activated B-cell humoral immunity. Clinical conditions that reflect increased Th2 responses include asthma and atopic syndromes as well as allergies. Research involving asthmatic subjects demonstrates a normal Th1 gene expression but a constant up-regulation of Th2-specific genes, leading to Th2 predominance.7 Although it is unclear what the cause of this exaggerated Th2 response is in asthma, it seems that genetics, fungi, heavy metal toxicities, nutrition, viruses, and pollution are factors in this up-regulation.8 The “hygiene hypothesis” is also gaining ground in the standard medical literature. It asserts that by minimizing exposure to infectious agents owing to lifestyle choices based on hygienic concerns, the dominance of Th2 immune responses to environmental allergens has been favored and thus also the probable encouragement of asthma and atopic diseases.9,10
Mediators
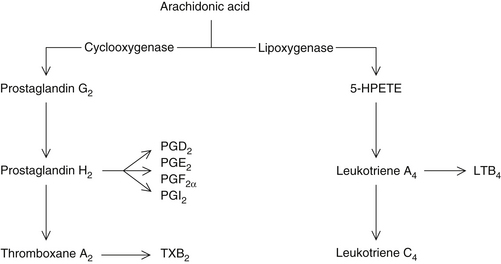
Both extrinsic and intrinsic factors involved primarily with Th2 imbalances trigger the cytokine-activated release of mast cell–derived chemical mediators. These mediators are responsible for bronchoconstriction, mucus production, and other signs and symptoms in the majority of cases. These mediators are either preformed within granules or generated from membrane-bound phospholipids. The preformed mediators include histamine, various chemotactic peptides such as eosinophilic chemolactic factor (ECF) and high-molecular-weight neutrophil chemotactic factor (NCF), proteases, glycosidases, and heparin proteoglycan. The membrane-derived agents include lipoxygenase products such as leukotrienes (LTs) and the so-called slow-reacting substance of anaphylaxis (SRS-A), prostaglandins (PGs), thromboxanes (TXs), and platelet-activating factor (PAF).
These mediators are diverse in chemical composition and modes of action and account for many of the signs and symptoms of asthma. Their actions include bronchial smooth muscle constriction (histamine, LTC4, LTD4 and LTE4, PGF2a, PGD2, and PAF), mucosal edema (increased permeability, histamine, LTC4, LTD4, and PAF), vasodilation (PGD2 and PGE2), mucous plugging (histamine, hydroxyeicosatetraenoic [HETE] acids, and LTC4), inflammatory cell infiltrate (NCF, ECF-A, HETEs, LTB4, and PAF), and desquamation of epithelium (proteases and glycosidases, together with lysosomal enzymes and basic proteins derived from neutrophils and eosinophils). In chronic asthma, eventual airway remodeling is a consequence of these mediator effects. The cellular and mediator elements responsible for airway remodeling involve the anomalous interface between the airway epithelium and the underlying mesenchymal tissues. This process is highlighted by the induction of growth factors that encourage fibroblastic and smooth muscle proliferation and the deposition of matrix proteins, which cause the thickening of the airway wall linked to bronchial hyperresponsiveness and fixed airflow obstruction.4
Lipoxygenase Products
The most potent chemical mediators in asthma are the lipoxygenase products (i.e., leukotrienes) (Figure 147-1). The leukotrienes composing SRS-A (LTC4, LTD4, LTE4) are 1000 times more potent as stimulators of bronchial constriction than histamine. Researchers have observed that asthmatics have an imbalance in arachidonic acid metabolism, leading to a relative increase in lipoxygenase products.11 Platelets from asthmatic patients show a 40% decrease in cyclooxygenase-derived metabolites and a 70% increase in lipoxygenase products. This pathophysiologic alteration is further aggravated in “aspirin-induced asthma.” Aspirin and other nonsteroidal anti-inflammatory drugs (e.g., indomethacin, phenylbutazone) inhibit cyclooxygenase while promoting lipoxygenase.12,13 The net result is a shunting of arachidonic acid toward the lipoxygenase pathway and the production of excessive levels of leukotrienes.
Tartrazine (yellow dye no. 5) is also a cyclooxygenase inhibitor and known inducer of asthma, particularly in children.12 Tartrazine is ubiquitous in processed foods and can even be found in vitamin preparations and antiasthmatic prescription drugs (e.g., aminophylline). Tartrazine may also indirectly support the asthmatic process via its role as an antimetabolite of vitamin B6 (see later discussion of tryptophan).
Autonomic Nervous System
The relationship between the autonomic nervous system and bronchial asthma represents an interaction between parasympathetic and sympathetic innervation and beta2 adrenergic receptors (which are localized in lung tissue and react to circulating catecholamines).14 Stimulation of the parasympathetic vagus nerve results in airway constriction. This vagal mechanism involves the following: release of acetylcholine at the synapse, subsequent binding to its receptor on smooth muscle tissue, and subsequent formation of cyclic guanosine monophosphate (cGMP). Accumulation of cGMP or a relative deficiency in cyclic adenosine monophosphate (cAMP), or both, result in constriction of the airway smooth muscle, as well as degranulation of mast cells and basophils. Decreased sympathetic activity or diminished beta2 receptor numbers or sensitivity also promote the cyclic nucleotide imbalance. Some of the mediators discussed earlier block beta2 receptors and elevate cGMP levels both directly and indirectly.
Pertussis Vaccine
An evaluation of health criteria among 448 children and adolescents in Britain who had received only breast milk for the first 6 months of life and in particular on the first day after birth produced some interesting findings. All of the children were weaned after 1 year of age and were older than 4 years at the time the parents responded. The mean age was 7.87 years. In response to the question “Has your child ever been diagnosed as asthmatic?” there were 30 positive answers (6.72%). The surprise came when the researchers classified the respondents according to whether or not they had received the pertussis vaccine.15
Influenza Vaccine
One evaluation of more than 9600 children was employed to determine the safety of cold-adapted trivalent intranasal influenza virus vaccine in children. Although this relatively new vaccination was deemed safe for children and adolescents, a significantly increased relative risk of 4.06 was observed in children 18 to 35 months of age for asthma and associated reactive airways disease.16
 Therapeutic Considerations
Therapeutic Considerations
General
Hypochlorhydria
Fractional gastric analyses in 200 asthmatic children by Bray in 1931 showed that 80% of them had gastric acid secretions below normal levels.17 This high occurrence suggests that decreased gastric acid output may predispose these children to food allergies and has a major impact on the success of rotation or elimination diets or both and, if not corrected, on the development of additional food allergies.
Increased Intestinal Permeability
The presence of food allergies is thought to be responsible for asthmatics having been shown to have “leaky guts.”18 As a result of increased gut permeability, there an is increased antigen load on the immune system. This subsequently overwhelms the immune system, increasing the likelihood of developing additional allergies as well as increasing bronchoconstrictive compounds in the circulation. It is essential to identify offending foods as soon as possible so as to avoid the development of further allergies.
Candida Albicans
An overgrowth of the common yeast Candida albicans in the gastrointestinal tract has been implicated as a causative factor in allergic conditions including asthma. Apparently the acid protease produced by C. albicans is the responsible allergen.19 Appropriate anticandidal therapy may result in significant clinical improvement of asthma in many cases.
Food Additives
Vitally important in the control of asthma is the elimination of food additives.20 Artificial dyes and preservatives are widely used in foods, beverages, and drugs. The most common coloring agents are azo dyes—tartrazine (orange), sunset yellow, amaranth, and coccine (red)—and the nonazo dye pate blue. The most commonly used preservatives in food are sodium benzoate, 4-hydroxybenzoate esters, and sulfur dioxide. Various sulfites are commonly used in prepared foods.
Tartrazine, benzoates, sulfur dioxide, and sulfites in particular have been reported to cause asthma attacks in susceptible individuals.20,21 It is estimated that 2 to 3 mg of sulfites are consumed each day by the average U.S. citizen, while an additional 5 to 10 mg are ingested by wine and beer drinkers.21 The largest sources are the salads, vegetables (particularly potatoes), and avocado dip served in restaurants. A customer can ingest 25 to 100 mg of metabisulfite in as little as one restaurant meal. It has been postulated that a molybdenum deficiency may be responsible for sulfite sensitivity.22 Sulfite oxidase, the enzyme responsible for neutralizing sulfites, is molybdenum dependent.
Salt
Strong evidence indicates that an increased intake of salt increases bronchial reactivity and mortality from asthma.23,24 The degree of bronchial reactivity to histamine is positively correlated with the 24-hour urinary sodium excretion and rises with increased dietary sodium. Because the severity of asthma correlates with the degree of bronchial reactivity, the severity of asthma can clearly be influenced by alterations in dietary sodium consumption.
Estrogen and Progesterone
For female patients who experience severe asthma symptoms, it may be useful to consider therapeutic regimens that encourage a decrease in hormonal fluctuations. Some studies find estrogen withdrawal to increase lung contractility.25 However, other studies find little relationship between estrogen use and asthmatic symptoms.26–28 Estrogen29 and progesterone are both known to act as smooth muscle relaxants. It may be that the airways of premenopausal and postmenopausal women may respond differently to exogenous hormone replacement and therefore need to be assessed independently with this intervention.
One interesting review of more than 90 papers published from 1966 to 2001 found that during the premenstrual and menstrual phases, when hormone levels are low, asthmatic women have been found to experience increased asthma exacerbations, increased hospitalizations for asthma, and decreased pulmonary function. Additionally, women who experienced decreased fluctuations in hormone levels either by becoming pregnant or by starting oral contraceptive therapy tended to have improvements in pulmonary function and fewer requirements for medication.25 Natural hormone replacement may offer a safer approach than synthetic hormones, although no studies to verify the safety or efficacy of these natural forms of female sex hormones have been conducted. In the longer term, natural therapeutics designed to balance estrogen and progesterone, along with specific recommendations from this chapter, may offer the most risk-free relief for those premenopausal and postmenopausal women who suffer from asthmatic exacerbations (see Chapter 188 and 202).
Dehydroepiandrosterone
Decreased levels of dehydroepiandrosterone (DHEA) have been shown to be common in postmenopausal women with asthma compared with matched controls.30 One study of 55 asthmatic and 20 healthy postmenopausal women aged 48 to 60 before hormone replacement therapy and after 6 months of transdermal 17b-estradiol (E2) and medroxyprogesterone acetate treatment demonstrated a significant increase in serum DHEA levels in the asthmatic group, with no change in the control group.31 Whether DHEA itself produces any therapeutic benefit in asthma remains to be demonstrated; however, given its important role in proper immune function, an ability to produce positive effects is certainly possible.
Melatonin
There is some concern regarding elevated melatonin levels contributing to increased airway inflammation in patients with nocturnal asthmatic exacerbations32,33 as well as proinflammatory reactions in rheumatoid arthritis.34 In the study of people with nocturnal asthma, peak measured serum melatonin level was significantly and inversely correlated with overnight change in FEV1 in subjects with nocturnal asthma but not in those with non-nocturnal asthma or healthy controls. An interesting note is that this study observed a delayed release of melatonin in the patients with nocturnal asthma. Therefore it is theoretically possible that supplementation combined with an earlier sleep regimen may help regulate this late peaking of melatonin and possibly mitigate symptoms. Nevertheless, the practitioner may want to avoid giving this supplement to patients with asthma, especially the nocturnal type, until more studies are conducted regarding melatonin’s immunotherapeutic effect.
Diet
Beneficial Foods
A number of publications corroborate the notion that people who have a diet rich in fruits and vegetables have a lower risk of poor respiratory health.35–37 This effect is most likely due to the increased levels of antioxidants in such foods. One epidemiologic review38 found that among children, consumption of fresh fruit, particularly fruit high in vitamin C, has been related to a lower prevalence of asthma symptoms and better lung function. This effect was observed even at low levels of fruit consumption (one or two servings per week vs. fewer than one serving per week), which suggests that a small increase in fruit intake could be beneficial. This same review discussed consumption of fish as also being related to lower airway hyperreactivity among children and better lung function in adults.
A study of Scottish adults also found a dose-response relationship between fruit consumption and pulmonary function, whereby increased fruit consumption led to decreases in phlegm and better pulmonary function.39 In addition, a “reasonable” intake of fruit (>180 g/day), whole grains (>45 g/day), and moderate alcohol consumption (one to three glasses a day) was associated with a 139-mL higher FEV1 and a 50% lower prevalence of chronic obstructive pulmonary disease versus diets that did not meet these intake requirements.35 Finally, one study of 607 asthma patients and 864 controls highlighted apples and moderate red wine consumption as sources of antioxidants that decreased asthma severity.40
Dietary intake of soy foods may be helpful, as the soy isoflavone genistein is associated with reduced severity of asthma. Although this effect may be due to some antioxidant action, studies have also shown that genistein is able to block eosinophil leukotriene C(4) synthesis and inhibit the pathway of NF-κB and TNF-α in asthma patients.41–43
Food Allergy
Many studies have indicated that food allergies play an important role in asthma (see Chapter 15.)44–48 Adverse reactions to food may be immediate or delayed. Double-blind food challenges in children have shown that immediate-onset sensitivities are usually due (in decreasing order of frequency) to egg, fish, shellfish, nuts, and peanuts, while foods most commonly associated with delayed onset include (in decreasing order of frequency) milk, chocolate, wheat, citrus, and food colorings.46 Elimination diets have been successful in identifying allergens and treating asthma and are a particularly valuable diagnostic and therapeutic tool in infants.49 Elimination of common allergens during infancy (first 2 years) has been shown to reduce allergic tendencies in high-risk children (e.g., strong familial history).50
Breastfeeding
Many studies have demonstrated the protective effect of breastfeeding in the prevention of asthma. One study examined the association of breastfeeding and the presence of chronic respiratory symptoms among 5182 Brazilian schoolchildren 7 to 14 years of age. Ninety percent of the mothers in this population had breastfed their infants. After adjusting for potential confounding factors, these researchers revealed that children who had not been breastfed were more likely to have a medical diagnosis of asthma.51 One Iraqi study additionally showed that the vitamin C content of breast milk was significantly correlated with the maternal intake of vitamin C: low vitamin C intake by mother translates to low intake by the child, thus leaving him or her more susceptible to oxidative stress.52
The Canadian Asthma Primary Prevention study collected 2 years of data in which researchers chose 545 infants who were considered at high risk for asthma on the basis of a history of familial atopy. These children were broken down into control and intervention groups. The interventions included (1) measures to control house dust; (2) recommendations for avoidance of pets, environmental tobacco smoke, and day care during the first year; and (3) only breastfeeding or use of partially hydrolyzed whey formula until at least the age of 4 months. At 1 year of age, risk of asthma was significantly reduced by 34%. At 2 years of age, significantly fewer children had asthma in the intervention group than in the control group (16.3% vs. 23%) and 60% fewer of those in the intervention group had persistent asthma. A 90% reduction in recurrent wheeze was seen in the intervention group compared with the control group.53 Multifactorial studies like these are quite useful in order to elucidate the multitude of changes necessary to affect multifactorial diseases like asthma in an effective manner.
Antibiotics, Probiotics, and Mucosal IgA
In a combined analysis of seven studies involving more than 12,000 youngsters, researchers at the University of British Columbia found that those prescribed antibiotics before their first birthday were more than twice as likely as untreated kids to develop asthma.54 And, if they had multiple courses of antibiotics it bumped up the risk even higher—16% for every course of the drugs taken before age 1. There are a couple of explanations for this association between antibiotic use and asthma. One is that antibiotics contribute to a state of “excess hygiene,” leading to a reduced exposure to microbes. This, in turn, creates an oversensitive immune system, mounting an over-the-top allergic reaction to pollen and dust mites and ultimately resulting in asthma. The second explanation is that antibiotics have a negative effect on the normal flora of the gastrointestinal tract and respiratory passages. Some studies have shown that giving probiotics (active cultures of Lactobacillus and Bifidobacterium species) lowers the risk of atopic allergic diseases like asthma and eczema. Some of this protective effect may be mediated by mucosal IgA, which participates in antigen elimination. In a cohort of 237 allergy-prone infants given a combination of four probiotic strains or placebo, it was shown that supplementation with probiotics increased fecal IgA and calprotectin while reducing inflammatory markers (e.g., α1-antitrypsin and tumor necrosis factor alpha).55 In infants with high fecal IgA concentration at the age of 6 months, the risk of having any allergic disease or any IgE-associated (atopic) disease before the age of 2 years was cut by nearly 50%. High intestinal IgA in early life is associated with minimal intestinal inflammation and indicates a reduced risk for IgE-associated allergic diseases.
Vegan Diet
A long-term trial of a vegan diet (elimination of all animal products) provided significant improvement in 92% of the 25 treated patients who completed the study (9 dropped out).56 Improvement was determined by a number of clinical variables including vital capacity, FEV1, and physical working capacity as well as by biochemical indices such as haptoglobin, IgM, IgE, cholesterol, and triglyceride levels in the blood. The researchers also found a reduction in the vulnerability to infection. Importantly, however, although 71% of the patients responded within 4 months, 1 year of therapy was required before the 92% level was reached.
The beneficial effects of this dietary regimen are probably related to three areas:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree