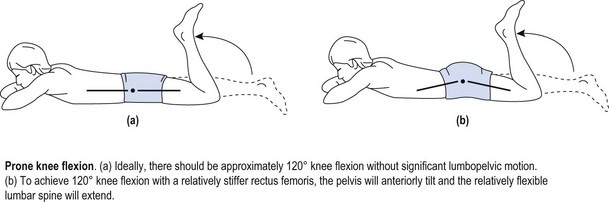
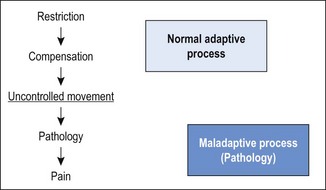
Chapter3 The development of valid classification methods to assist therapists in the management of neuromusculoskeletal disorders has been recognised as a clinical priority (Fritz & Brennan 2007; Fritz et al 2007). Identifying and classifying movement faults is fast becoming an essential tool in contemporary rehabilitative neuromusculoskeletal practice (Comerford & Mottram 2001a; Sahrmann 2002; O’Sullivan 2005). Traditionally, assessment of musculoskeletal problems is based on the clinical history, mechanism of injury and symptom responses to examination procedures. Symptoms are assessed during active movements (Cyriax 1980; McKenzie & May 2003; Maitland et al 2005), passive movements (Kaltenborn 2003; Maitland et al 2005), combined movements (Edwards 1999) or sustained positions (McKenzie & May 2003). A mechanism-based approach has now been proposed (Schafer et al 2007) with contemporary assessment moving away from individual symptom responses to exploring movement impairments and how these relate to symptoms (Comerford & Mottram 2001a; Sahrmann 2002; Burnett et al 2004; Dankaerts et al 2006b; Comerford & Mottram 2011, Van Dillen et al 2009). Given the complexity of neuromuscular dysfunction, therapists have continued to search for a systematic framework to assist clinical assessment and management. One focus is on identifying clinical prediction rules (CPR) that determine subgroups within patient presentations that may respond to certain treatments (Hicks et al 2005); however, it has yet to be established if CPR can change symptoms as well as function and dysfunction or correlate to changes in muscular recruitment. The following section explores issues relating to the classification of subgroups in neuromusculoskeletal pain management. Non-specific musculoskeletal pain often has a history of chronicity or recurrence along with multiple tissues being diagnosed as contributory elements to the pain presentation. Significant pain mechanisms are often present (Chapter 1) and there may or may not be identifiable elements of behavioural adaptation. If mechanical subgroups can be identified within the broad group known as non-specific neuromusculoskeletal pain, then manual therapy and therapeutic exercise interventions have a better rationale for predicting positive outcomes. • Non-specific musculoskeletal pain: no single anatomical based pathology can account for the presenting symptoms. The evaluation of movement-related dysfunction can be used to explain some of the symptoms presenting in multiple tissues. These movement-based dysfunctions include the evaluation of the site and direction of uncontrolled movement (UCM), recruitment efficiency of local muscle stability system, muscle imbalance, patterns of movement provocation and relief with postural positioning, positional diagnosis, patterns of symptom relief associated with manual mobilisation. Box 3.1 illustrates some of these subgroups. • Specific musculoskeletal pain – classification by implying a patho-anatomical source: definite pathology is identified that accounts for the presenting signs and symptoms, for example: spondylolisthesis, disc herniation and nerve root compression, spinal stenosis, bony injury/fracture, articular derangement (meniscal/labral tear, chondral defect), muscle haematoma and osteoligamentous damage (ligament sprain). • Classification by pain mechanisms in particular identifying components of inflammatory/biochemical sensitisation, neurogenic sensitisation and behavioural or psychosomatic issues (Watson & Kendall 2000; Butler & Moseley 2003; Sterling et al 2003, 2004; Waddell 2004). In the absence of reliable diagnostic tests for musculoskeletal disorders, classifying movement control faults is gaining recognition and acceptance (Comerford & Mottram 2001a; Sahrmann 2002; Dankaerts et al 2006b; Mottram & Comerford 2008). For example, identifying sub-categories of movement faults to guide interventions has been applied to the lumbar spine and the reliability of some tests has been established (Luomajoki et al 2007; Trudelle-Jackson et al 2008). Comerford & Mottram (2001a, 2011) contend that the observation of aberrant movement in itself may not be the most critical factor influencing pain and dysfunction. It could be argued that some observations of excessive or reduced range of movement may just be variations within the normal distribution of the population. People who have no pain and no history of previous symptoms may present with range of motion that may be considered excessive or hypermobile. It is possible that this ‘excessive’ range of movement is controlled well by automatic and cognitive recruitment mechanisms during movement and postural tasks (Roussel et al 2009). The ability to cognitively recruit appropriate movement control strategies may be a better indicator of whether there is UCM or whether the aberrant movement is merely a bad habit at one extreme of the normal distribution curve. Not only is the observation of aberrant movement important but it is important to be able to test for the ability to control it. Sahrmann (2002) proposes the concept of ‘relative flexibility’ or ‘relative stiffness’. If one-joint muscles become excessively lengthened and strained or are ‘weak’ and lack the ability to adequately shorten, they demonstrate increased flexibility. This increased flexibility can contribute to uncontrolled or excessive motion at that joint. Similarly, if multi-joint muscles lack extensibility or generate excessive tension they develop increased stiffness. This increased stiffness then has the potential to limit or restrict normal motion at that joint. When increased stiffness limits motion at a joint, then in order to maintain normal function, the restriction must be compensated for elsewhere in the movement system. If these muscles are linked in functional movements then excessive or uncontrolled motion develops at the joint that is inadequately controlled by the one-joint muscles relative to the adjacent restriction. Relatively more flexible structures compensate for relatively stiffer structures in function, creating direction-specific stress and strain. During functional movements direction-specific hypermobility is re-enforced and if repetitively loaded, tissue pathology results (Comerford & Mottram 2001a). An example of this concept can be observed in the active prone knee extension test (Woolsey et al 1988). If the rectus femoris is relatively stiffer than the abdominals, then in order to achieve 120° of knee flexion, the pelvis tilts anteriorly, and the spine extends. Sahrmann (2002) suggests that the abdominals are relatively more flexible than the rectus femoris, which is relatively stiffer, creating uncontrolled or abnormal spinal extension, which in turn contributes to mechanical back pain (Figure 3.1). Sahrmann (2002) also identified a similar pattern during forward bending manoeuvres. If the hamstrings are relatively stiffer than the back extensors (which are relatively more flexible), then during forward bending the hip lacks sufficient flexion but the spine hyperflexes to compensate. This may predispose to mechanical back pain. Esola et al (1996) reported that subjects with a history of low back pain, in early forward bending, flex more at their lumbar spine and have stiffer hamstrings than do subjects with no history of low back pain. This is supported by Hamilton & Richardson (1998) who show that subjects who have no low back pain can actively maintain spinal neutral alignment through 30° of forward leaning (hip flexion) in sitting, but subjects with low back pain cannot. The low back pain subjects lost neutral alignment earlier and to a greater extent, indicating that the spine was relatively more flexible than the hips in low back pain subjects. Similar evidence has been reported in cervical spine dysfunction. The normal ranges of segmental flexion–extension range of motion for C5–6 is 18° and 17° for C4–5 with 3.2 mm of intersegmental translation at both levels (Bhalla & Simmons 1969; Dvorak et al 1988). Singer et al (1993) reported that subjects with neck pain and discogenic pathology demonstrated changes in range of segmental motion and intersegmental translation. The C5–6 motion segment became relatively stiff. It demonstrated reduced range of flexion–extension from 18° to 8° and intersegmental translation reduced from 3.2 mm to 1 mm. In order to maintain functional range of motion of the head and neck, the C4–5 motion segment increased flexibility. It demonstrated increased range of flexion–extension from 17° to 23° and intersegmental translation increase from 3.2 mm to 6 mm. This paper demonstrated that a significant restriction of motion at one vertebral level could be compensated for by relatively increasing range at an adjacent level. Norlander & Nordgren (1998) suggest that deviation from synchronous distribution of normal mobility between motion segments might be a factor causing provocation of joint mechanoreceptors and subsequent pain. They measured segmental relative flexion mobility between C5 and T7 and identified that hypomobility of C7–T1 with hypermobility of T1–2 significantly predicted neck–shoulder pain. Relative stiffness/flexibility changes have also been measured at the shoulder girdle. Sahrmann (1992, 2002) identifies several clinical patterns of dysfunction. Increased glenohumeral motion compensates for insufficient upward rotation of the scapula during shoulder flexion or abduction. Increased forward tilt of the scapula compensates for shortness or stiffness of the lateral rotator muscles during shoulder medial rotation. Increased anterior translation of the humeral head compensates for restriction of glenohumeral medial rotation. She further suggests that these compensations are associated with the development of pathology. A test of shoulder girdle relative stiffness/flexibility (the kinetic medial rotation test – Chapter 8) identifies a restriction of shoulder medial rotation, which is compensated for by relatively increasing scapular forward tilt or glenohumeral translation to maintain a functional range of arm rotation. It is suggested that the compensatory motion at the scapula correlates with impingement pathology, while glenohumeral compensatory motion correlates with instability pathology. This test has been further validated and quantified by Morrissey (2005) and Morrissey et al (2008). A common feature of movement control faults is reduced control of active movements, or movement control dysfunction, termed MCD by Luomajoki et al (2007). The MCD is identified by a series of clinical tests. These tests have been shown to be reliable in the lumbar spine (Luomajoki et al 2007; Roussel et al 2009) and have been promoted in clinical practice (Mottram 2003; Comerford & Mottram 2011). The tests are based on the concept known as dissociation, defined as the ability to control motion at one segment while concurrently producing an active movement at another joint segment (Comerford & Mottram 2001a; Sahrmann 2002). A dissociation test evaluates the ability to actively control movement and demonstrates MCD. Once a MCD has been identified it can guide the choice of therapeutic exercise (Comerford & Mottram 2001b; Mottram 2003). In the case of shoulder dysfunction, muscles around the shoulder girdle may be unable to control the scapula during arm function. In the lumbar spine, trunk muscles may be unable to control lumbar alignment during movements of the hip or thoracic spine. The distinctive features of these tests start with the positioning of the spine or segment in its ‘neutral position’ by the therapist, which is then actively controlled by the patient while they move the joint region either above or below the joint system being tested. These clinical dissociation tests can identify the site (e.g. scapula or lumbar spine) and direction (e.g. downward rotation/forward tilt, and flexion) of movement control faults (Luomajoki et al 2008; Barr & Burden 2009; Mottram et al 2009). Adapting the principles associated with dissociation testing, UCM can be identified and classified by the therapist using palpation and visual observation. These clinical tests are described in Chapters 5–9. A standardised clinical examination, based on Sahrmann’s conceptual model of movement impairment, has been described for the lumbar spine (Scholtes & Van Dillen 2007; Van Dillen et al 2009), the knee (Harris-Hayes & Van Dillen 2009) and the shoulder (Caldwell et al 2007). The underlying assumption is that movement faults and abnormal resting postures are associated with musculoskeletal tissue changes (Sahrmann 2002). For example, muscle dysfunction in relation to: i) muscle length changes; ii) altered recruitment patterns between synergistic or antagonistic muscles; and iii) direction specific increased motion which arises as compensation for relative restrictions of motion at adjacent joints may be determined. Movement system impairments (MSI) may present as abnormal alignment and impaired movement during testing or functional activities (Sahrmann 2002; Trudelle-Jackson et al 2008; van Dillen et al 2009). The lumbar spine examination includes a number of clinical tests of trunk, limb or combined trunk and limb movements to ascertain movement impairments (Van Dillen et al 1998, 2009). The MSI diagnosis is based on identifying, firstly, a consistent pattern of movement which is associated with the patient’s symptoms and, secondly, a decrease in pain when the MSI is corrected. For the lumbar region the clinician makes a judgment as to whether the patient moves his or her lumbopelvic region early in the test. For example, in a forward bending movement it may be observed that the lumbar spine initiates the forward bending movement, with hip flexion contributing to the forward bending much later. The person usually notes that their symptoms are provoked by and are linked to the lumbar flexion phase of the movement. The therapist also observes whether a significant reduction in the symptoms is achieved if the person can learn to initiate forward bending with hip flexion, while actively preventing the lumbar spine flexion. On this basis a diagnosis of lumbar flexion movement impairment is made. People with low back pain (LBP) demonstrate early lumbopelvic movement with clinical tests (Scholte et al 2000; Gombatto et al 2007; van Dillen et al 2001 2009). The inter-rater reliability between two physical therapists classifying patients with chronic LBP into lumbar spine movement impairment strategies has substantial agreement (Trudelle-Jackson et al 2008). The suggestion is that this links to the pattern of movement during everyday activities and relates to LBP. The hypothesis here is that early lumbopelvic movement during everyday activities suggests an increase in frequency of movement of a specific region which may contribute to increased stress on tissue resulting in pain (Mueller & Maluf 2002). This becomes the diagnosis of movement impairment. O’Sullivan (2000) proposed a classification system based on motor control impairments (MCI). His classification system of clinical subgroups is based on altered strategies for postural and movement control. The inter-tester reliably of this classification system has been established (Vibe Fersum et al 2009). O’Sullivan describes a subgroup of patients presenting with impairments in control of spinal segments in the direction of pain which are associated with deficits in motor control (O’Sullivan et al 2006). Interestingly, Dankaerts (2006a), in applying this system, did not identify differences in superficial trunk muscle activation between a group of healthy controls and non-specific chronic LBP subjects in sitting. The authors stressed the importance of the ‘washout effect’ when interpreting this finding. When results from all subjects with chronic LBP were pooled the findings in one subgroup of patients were ‘washed out’ by the others. However, once subjects were grouped by flexion and extension control impairment patterns, clear differences in muscle activation patterns were identified. The identification of UCM should be made in terms of site and direction based on the ability to cognitively control the movement, not just on observation of altered range of motion. The consideration that a significant amount of pain in the neuromusculoskeletal system is a result of cumulative microtrauma caused by uncontrolled movement is gaining credibility (Sahrmann 2002; Luomajoki et al 2007; Van Dillen et al 2009). The uncontrolled motion leads to increased loading and pain (Cholewicki & McGill 1996; Mueller & Maluf 2002). UCM is not identified by merely noting hypermobile range of motion or relative flexibility. Furthermore, UCM is not solely identified by habitual postures or initiation of function with movement at one segment. UCM is identified by a lack of the ability to actively control or prevent movement (or lack of ability to learn how to control movement) in a particular direction at a particular joint or motion segment. The UCM can be identified in the presence or in the absence of a symptomatic episode. The UCM is independent of hypermobile or hypomobile range of motion. That is, some people may demonstrate UCM even in situations of reduced functional range, while other people with hypermobile range of motion may demonstrate good active control of their excessive range of motion. The presence of UCM is a powerful indicator of symptomatic function associated with recurrence and chronicity of musculoskeletal pain. The development of restrictions within normal motion is common. The body acquires restrictions over time for a variety of reasons, as described in Box 3.2. Motion restrictions may be passive or active, affecting either the accessory translation or the physiological range available to a joint. Passive restrictions may involve: i) a loss of extensibility of normal contractile structures (e.g. muscle shortening); ii) connective tissue structures (e.g. capsule shortening); iii) the development of abnormal connective tissue (e.g. fibrotic adhesions); or iv) bony changes (osteophytes or spurs) that contribute to a reduction of available passive joint motion. Active restrictions may involve neurally mediated changes in contractile (muscle) tissues. This may occur as a result of: i) muscle guarding or spasm in response to pain sensitive movement; or ii) increased muscle tension/stiffness due to altered patterns (strategies) of muscle recruitment between synergistic muscle groups or increased muscle tension in response to emotional, behavioural or environmental stressors. These altered patterns of muscle recruitment may in turn be reinforced due to overuse, overtraining, postural loading or maladaptive responses to pain, stress and psychosocial factors. Because restrictions of normal motion are common, the body normally compensates for these restrictions by increasing motion elsewhere to maintain function. In normal functional movement, the central nervous system (CNS) has a variety of strategies available to perform any functional task or movement and, ideally, the CNS determines the most appropriate strategy for the demands of the functional task. So long as the trajectory or path of motion is well controlled by the coordination of forces in the local and global synergists, the movement system appears to cope well (Hodges 2003). Compensation that demonstrates effective active control is a normal adaptive process and does not constitute a stability dysfunction, and is usually non-symptomatic. However, inefficient active control (uncontrolled movement) identifies a dynamic stability dysfunction and has greater potential to accumulate microtrauma within a variety of tissues and if this exceeds tissue tolerance may contribute to the development of pathology and pain (Comerford & Mottram 2001a) (Figure 3.2). UCM is defined as a lack of efficient active recruitment of the local or global muscle’s ability to control motion at a particular motion segment in a specific direction (Comerford & Mottram 2001a). For example, uncontrolled lumbar flexion demonstrates a lack of efficient active recruitment of spinal muscles to control or prevent movement of the lumbar spine into flexion when attempting to do so. The development of UCM may have several contributing factors: 1. Compensation for restriction to maintain function. The UCM most commonly develops insidiously to compensate for an articular or myofascial restriction in order to maintain normal function. This is commonly observed as lack of control of hypermobile range; however, it can also present as a lack of control of normal range. For example, uncontrolled lumbar flexion compensates for a restriction of hip flexion (hamstrings) to maintain the normal function of forward bending. The back extensor stabiliser muscles lack efficient control of the lumbar spine during flexion loading. Therefore, the UCM is in the lumbar spine in the direction of flexion. 2. Direct overfacilitation. Occasionally the UCM develops because excessive range of movement is habitually performed (without compensating for restrictions). A particular muscle pulls too hard on a joint in a particular direction due to dominant recruitment, active shortening or overtraining. This develops slowly as a progressive insidious process. This is due to an active process of overuse and shortening of a particular muscle that holds a joint towards its end-range position (away from neutral or mid-range positions). For example, uncontrolled lumbar flexion develops due to overtraining of rectus abdominis with repetitive trunk curls. Rectus abdominis actively holds the lumbar spine excessively flexed at rest and during flexion load activities and postures. The back extensor stabiliser muscles lack efficient control of the lumbar spine during flexion loading. Therefore, the UCM is in the lumbar spine in the direction of flexion.
Assessment and classification of uncontrolled movement
Classification of subgroups in neuromusculoskeletal pain
Classification based on movement dysfunction
Relative stiffness – relative flexibility
Movement control dysfunction
Movement impairments
Motor control impairments (MCI)
Uncontrolled movement (UCM) and pain
The development of motion restrictions in function
A proposition for the aetiology of UCM
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree