Arboviruses and Related Zoonotic Viruses
Theodore F. Tsai
Michael Bell
The arboviruses (arthropod-borne viruses) are a heterogenous group of more than 500 viruses classified into 13 taxonomic families (Table 184.1). Despite their taxonomic diversity, the vector-borne and certain related zoonotic viruses are studied together because of their common natural reservoirs and overlapping modes of transmission. Table 184.2 lists these diseases, arranged by the principal clinical manifestation of infection, mode of transmission, and the frequency with which the disease
has been recognized. Several of these diseases are relatively uncommon, with only one or two cases of human infection reported, although antibody prevalence studies may suggest the possibility of more widespread infection.
has been recognized. Several of these diseases are relatively uncommon, with only one or two cases of human infection reported, although antibody prevalence studies may suggest the possibility of more widespread infection.
TABLE 184.1. CHARACTERISTIC OF ARBOVIRUSES AND CERTAIN ZOONOTIC VIRUSES | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
The number of these agents may appear daunting, but a careful evaluation of the clinical syndrome, combined with the appropriate use of a reference diagnostic laboratory, can help to identify the etiology of infection in most cases. In addition to the syndromic and laboratory features of the illness, epidemiologic factors in the patient’s history should be sought to identify contact with or potential exposure to an infectious source or vectors (e.g., appropriate season and place of exposure within the relevant incubation period preceding onset of illness). Many of the known arthropod-borne and related zoonotic viruses are orphan viruses with unknown disease potential; additionally, novel viruses and viral-disease associations continue to be identified as human incursions increase into rodent and other vector habitats.
Although the recognized arboviruses are distributed principally in developing countries, “tropical” infections are encountered increasingly in returned travelers and no longer can be considered exotic. Dengue fever, probably the arboviral infection most frequently acquired during travel, is diagnosed in approximately 50 travelers returning to the United States each year. Yet these cases undoubtedly represent only a small fraction of the true number of cases because of the illness’s nonspecific presentation and because adequate diagnostic specimens are not obtained routinely. Examples of other arboviral infections imported by travelers include Mayaro fever acquired in Central and South America; Toscana and Central European tickborne encephalitis (CEE) from Europe; West Nile–associated viral myelitis from the Middle East; viral retinitis associated with Rift Valley fever (RVF), Lassa fever, Thogoto viral encephalitis, and Ilesha viral infections from Africa; Ross River viral arthropathy from Australia; and chikungunya infection and Japanese encephalitis (JE) from Asia. Cases of fatal yellow fever occurring in nonimmunized travelers underscore the importance of providing pretravel counseling and appropriate immunizations.
Several arboviral infections indigenous to the United States remain important to the public health, most notably West Nile encephalitis (WNE), which has spread at a remarkable pace in epidemics across the North American continent since its introduction in 1999. LaCrosse encephalitis is an important pediatric disease in areas of the Midwest, where its incidence exceeds that of herpes encephalitis and, in some locations, equals that of Haemophilus influenzae meningitis in the preimmunization era. The expansion of residential development into wooded locations and the increasing popularity of outdoor recreation in remote areas have been associated with the increased occurrence of numerous bacterial, rickettsial, or protozoal vector-borne diseases such as Lyme disease, ehrlichiosis, and babesiosis. These same trends also have led to increased transmission of viral infections, such as hantavirus pulmonary syndrome—now with confirmed cases in 31 states; Eastern equine encephalitis (EEE), which occurs in eastern marshlands; and Colorado tick fever, which is distributed chiefly in the Rocky Mountain states.
EPIDEMIOLOGY
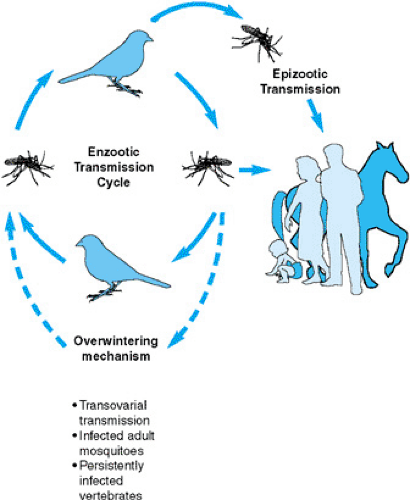
Most arboviruses are maintained in natural cycles of infection between vector insects or ticks and vertebrate hosts, with seasonal variations in cycle amplitudes related to extremes of humidity, temperature, and other environmental factors. The viruses may be carried through the winter or dry months by vertical transovarial transmission in vector mosquitoes, by transtadial transmission in ticks (across tick developmental stages, such as larval to nymphal to adult stage), or in persistently infected vertebrates; in tropical locations, transmission may occur continuously. A common pattern of enzootic transmission in a bird–mosquito cycle is illustrated in Figure 184.1. Human infections are most likely to occur in circumstances of intense viral transmission, when the virus “spills over” from the enzootic cycle. The enzootic foci of certain “place-specific” diseases, such as sylvatic yellow fever and Venezuelan equine encephalitis, may be maintained in relatively remote or inaccessible locations, so that humans are infected when they impinge on these otherwise isolated locations. Epizootic or bridging vectors are required to transmit infection to humans or other dead-end hosts in such diseases as EEE, for which the enzootic vector exhibits a narrow “biting preference” limited to the amplifying host. A different vector, therefore, is needed to deliver the virus to humans. Vertebrate-amplifying hosts must sustain viremias of sufficient titers (e.g., 104 pfu/mL or higher) and for sufficient periods (e.g., 3 to 5 days) to provide sufficient opportunity for enzootic vectors to become infected. Certain arboviruses, notably yellow fever, dengue, chikungunya, and Oropouche viruses, produce sufficient viremias in humans that anthroponotic (vector-borne interhuman) transmission is possible, with the possibility of pure human-driven epidemics occurring. In Africa, yellow fever epidemics arise when viremic persons traveling from areas of enzootic virus transmission introduce the virus into urban areas, where peridomestic breeding Aedes aegypti mediate a human–mosquito–human cycle.
The transmission cycles of many arboviruses are complex and not understood fully. (More detailed characterizations of the individual viruses are given in the Suggested Readings.) Several of the diseases mentioned in this chapter are zoonoses in which infections arise through direct contact with infectious material (e.g. blood, excreta, respiratory secretions) from a reservoir host. The arenaviruses, which cause Lassa and other hemorrhagic fevers and lymphocytic choriomeningitis, and the hantaviruses are spread from persistently infected rodents to humans. Human infection acquired from bats with Rio Bravo virus, Duvenhage virus, and Issyk-kul virus, and from shrews with Mokola virus are examples of uncommon viral zoonoses. Omsk hemorrhagic fever has been transmitted from infected muskrats to trappers during skinning, and RVF and Congo-Crimean hemorrhagic fever (CCHF) from infected livestock to herders and to butchers during slaughter, as well as by contact with the infectious ticks. Person-to-person transmission of CCHF and Ebola, Marburg, and Lassa viruses has led to sizable outbreaks associated with high mortality rates (some greater than 90%). Person-to-person transmission is facilitated in settings or by occupations associated with very close contact, especially health care delivery.
DIAGNOSIS
Effective clinical diagnosis of these viral infections requires a detailed case history to determine the patient’s possible exposures and their relationships to established incubation periods for these infections. In general, arboviral infections are acute and preceded by an incubation period ranging from several days to no more than several weeks. A longer interval suggests an alternate diagnosis. A detailed list should be made of the dates and places of travel, the kinds of habitats encountered, dwellings occupied, and the activities in which the patient was engaged, in addition to any history of military service and residence in tropical areas (where previous asymptomatic infections may have been acquired). The evaluation should include a careful assessment of immunization history, including yellow fever, JE, or other arboviral vaccines.
Most arboviral infections produce a nonspecific febrile illness, and specific laboratory tests are needed to confirm their diagnosis. Often, patients are seen after the acute phase of illness, and serology is the only means by which the diagnosis can be made; however, several arboviruses, including those of dengue, chikungunya, yellow fever, Venezuelan equine encephalitis, and Colorado tick fever, can circulate in the blood for several days, whence they can be recovered in the acute febrile phase of the illness. Suckling mice, primary duck embryo cells, and continuous mosquito cell lines (e.g., C636) are especially sensitive systems for recovering most arboviruses, but Vero cells, which are more widely available, are an adequate system for viral culture in most cases. The intrathoracic inoculation of Toxorhynchites mosquitoes is a sensitive system for the isolation of dengue and yellow fever viruses. Neurotropic arboviruses should be sought by real-time polymerase chain reaction (PCR) of cerebrospinal fluid (CSF). Viral culture, immunohistochemical studies, or electron microscopy should be undertaken when a brain biopsy is obtained from patients with suspected viral encephalitis.
In most instances, the detection of viral-specific IgM in serum is the most rapid approach to making a presumptive laboratory diagnosis. Its detection in the CSF, reflecting an intrathecal antibody response, is diagnostic of acute central nervous system (CNS) infection, whereas IgM in a single serum specimen provides only a presumptive diagnosis, because specific IgM may persist beyond a single transmission season in some individuals. Infection is confirmed serologically by demonstrating seroconversion (i.e., fourfold or greater change in titer) in appropriately timed serum specimens. The diagnosis is considered presumptive if a high titer is present in a serum pair or in a single serum specimen. Indirect immunofluorescence (IF) is used widely to measure IgM and IgG antibodies. Although IgG assays are sensitive, serologic cross-reactions to viruses within a taxonomic group frequently are encountered. The hemagglutination inhibition (HI) test, using goose erythrocytes, is a sensitive but broadly reactive assay for those arboviruses that exhibit the property of hemagglutination. Heterologous reactions may be encountered in patients previously infected by related viruses. The complement fixation (CF) test is more specific but is not entirely sensitive, because the antibody response is slow and unpredictable, and it can result in false-negative results. Although, in general, neutralization tests provide the highest specificity, they are inconvenient to perform.
The direct detection of viral genomic material or antigens in blood or other fluids is an important alternative to serology in diagnosing Ebola and other hemorrhagic fevers. Circulating antibodies against theses viruses may not be detectable for several weeks, and infection may cause death before the patient has had time to develop detectable antibodies. Establishing the diagnosis rapidly in the acute phase of illness is important for timely decisions to be made regarding both therapeutic management of the individual patient and public health and infection-control requirements to prevent additional cases. PCR analysis of acute serum samples is a sensitive and specific approach to laboratory diagnosis of dengue fever, Venezuelan equine encephalitis, O’nyong nyong, Ebola, and other infections producing relatively high viremia levels. In addition, viral particles can be visualized by electron microscopy on tissue culture supernatant after successful viral culture, offering useful information for several pathogens with distinctive morphologies. Viral antigens also can be visualized directly by immunohistochemistry in fixed or frozen tissues.
Serologic diagnostic testing is available through several private diagnostic laboratories, state health department laboratories, some university laboratories, and the U.S. Army Medical Research Institute of Infectious Diseases. Assays for all these agents, including viral isolation and viral antigen or genomic detection, are available after consultation from the Centers for Disease Control and Prevention (CDC). Until the specific etiology of a viral hemorrhagic fever is determined, all testing is performed best in a biosafety level 4 facility, because most of these agents are aerosol-infectious in a laboratory setting.
Cases of arboviral encephalitis should be reported to state health departments. Available public health control measures can reduce their transmission and epidemic threat. Because of the possibility of nosocomial transmission occurring and the availability of diagnostic assays, all suspect cases of viral hemorrhagic fever should be reported to the Special Pathogens Branch, CDC. Until the specific etiology of a viral hemorrhagic fever is determined, all testing should be performed in a biosafety level 4 (BSL-4) because of the high risk of occupational laboratory transmission occurring and the possibility of person-to-person spread of some of these viruses. High-risk laboratory procedures, such as centrifugation, which can generate infectious aerosols, require special precautions. All wastes and exhaust from BSL-4 facilities are sterilized before leaving the facility. Inactivated aliquots of a specimen can be removed from the BSL-4 facility for further assessment. Because of the transmissibility and potential severity of illnesses caused by many of these pathogens, appropriate reporting of cases is an important aspect of diagnosing. The laboratory diagnoses of arboviral encephalitis should be reported to the state health department so that public health measure (e.g., vector control) can be applied to reduce the risk of further transmission and a possible epidemic. Any suspected case of viral hemorrhagic fever poses a high risk of health care–associated transmission
(e.g., to other patients, health care personnel, and laboratory personnel) and should be reported immediately to the viral Special Pathogens Branch, CDC, for appropriate diagnostic assessment.
(e.g., to other patients, health care personnel, and laboratory personnel) and should be reported immediately to the viral Special Pathogens Branch, CDC, for appropriate diagnostic assessment.
Differential Diagnosis
Most arboviral infections lead to nonspecific illnesses consisting of fever, headache, musculoskeletal aches, and malaise, which cannot be differentiated clinically from the prodrome of viral hemorrhagic fevers or from a wide variety of viral bacterial and parasitic infections. The presence of rash can be somewhat helpful in guiding clinical diagnosing, although a lack of specificity limits its utility. Dengue and West Nile fever, certain other arboviral infections, Lassa fever, and filoviral hemorrhagic fevers can cause a morbilliform exanthem. Frequently, dengue has been misdiagnosed as measles or rubella, because these diseases also appear in outbreaks. The rash can be evanescent and difficult to detect in dark-skinned persons. A maculopapular rash can be seen in infections caused by enteroviruses, human parvovirus, rhinoviruses, reoviruses, parainfluenza viruses, rotavirus, hepatitis B virus, Epstein-Barr virus (EBV), cytomegalovirus (CMV), human herpesvirus 6, various rickettsia, and Mycoplasma pneumoniae infections. Scarlet fever, leptospirosis, relapsing fever, and medication-associated eruptions also can cause maculopapular rashes. Petechial eruptions can be seen in dengue, Colorado tick fever, alphavirus infections, and the hemorrhagic fevers, and these eruptions may appear similar to those seen in tickborne typhus, epidemic or murine typhus, meningococcemia, Brazilian purpuric fever, bacteremia from H. influenzae, certain enteroviral infections, and Henoch-Schönlein purpura. Sindbis and related alphaviral infections can produce a rash and polyarthritis resembling rubella. Their differential diagnosis also includes fifth disease, hepatitis A and B, EBV and certain enteroviral infections, M. pneumoniae infection, serum sickness, rat-bite fever, acute rheumatic fever, enteroarthritis, rheumatoid arthritis, and other infections producing acute symmetric polyarthritis.
Dengue, yellow fever, RVF, and CCHF viruses frequently produce a mild to moderate hepatitis, and in endemic areas, they should be considered in the differential diagnosis of ordinary viral hepatitis.
Arboviral CNS infections are not clinically distinctive, although a focal neurologic presentation is atypical. Focal signs suggestive of herpes encephalitis have been reported in isolated cases of western and snowshoe hare viral encephalitis, and they are a feature in approximately 25% of children with Powassan and La Crosse encephalitis. In addition, other treatable CNS conditions that should be ruled out are a partially treated bacterial meningitis, M. pneumoniae infection, Rocky Mountain spotted fever, leptospirosis, tuberculosis, Lyme disease, listeriosis, typhoid fever, cat-scratch encephalopathy, fungal meningitis, cerebral malaria, toxoplasmosis, cysticercosis, lead or other toxic encephalopathies, and heat stroke. An important consideration in the differential diagnosis, because a history of exposure to mosquitoes may be given, is encephalopathy from N, N-diethyltoluamide (DEET), which presents with encephalopathic signs and CSF pleocytosis. Rabies should be retained in the differential diagnosis even if no history of animal bite is given, because such exposures often are overlooked (especially in the case of bat exposure), the incubation period is variable, and hydrophobia may not be prominent.
The seasonal distribution of arboviral encephalitis cases in late summer and early autumn may be an important clue to the diagnosis, although this seasonality overlaps that of enteroviral infections, which are the leading cause of meningoencephalitis in this interval. Other viral causes of meningoencephalitis include infections from mumps virus and its vaccine, EBV, CMV, adenoviruses, influenza virus, and postinfectious encephalitis from the viruses associated with childhood exanthems. Polio may be encountered in unvaccinated persons and in immunocompromised persons exposed to vaccine strains. Infection with human immunodeficiency virus (HIV) type 1 may present with CNS signs before symptoms of systemic illness are noticed.
Other disorders of the CNS that should be considered in the differential diagnosis of meningoencephalitis include sarcoidosis, reactions to trimethoprim-sulfamethoxazole and other medications, vascular disorders of the CNS, space-occupying lesions, trichinosis, and CNS infections from free-living amoebae and Baylisascaris procyonis. Hemorrhagic shock–encephalopathy syndrome should be considered in the differential diagnosis for infants.
THERAPY
Research on therapeutic modalities has focused on the treatment of the hemorrhagic fevers because of their high case-fatality rates. In controlled trials of adults, ribavirin reduced mortality and morbidity rates in Lassa fever and hemorrhagic fever with renal syndrome (HFRS), and anecdotal experience suggests possible efficacy in Argentine hemorrhagic fever and CCHF. Immune plasma is an effective therapy for Argentine hemorrhagic fever, and passive immunization has been used empirically in the therapy or prophylaxis of other hemorrhagic fevers and after laboratory exposure to certain arboviruses. Immune globulins are available for CCHF and for tickborne encephalitis (TBE), but use of the latter in post-exposure prophylaxis and treatment of TBE has been associated with exacerbated illness in some cases. Although similar adverse experiences have not been reported in a limited number of JE and WNE cases treated with passive immunization, caution should be exercised. In one controlled trial, interferon alpha-2a therapy failed to improve the outcome of JE cases.
Supportive therapy for patients with encephalitis necessitates close attention to monitoring intracranial pressure (ICP), cardiorespiratory function, and fluid and electrolyte balance. Inappropriate secretion of antidiuretic hormone and hyponatremia is a frequent complication of arboviral encephalitis. Although patients with EEE, JE, and other arboviral infections of the CNS frequently exhibit clinical signs of elevated ICP, one controlled study of JE demonstrated that dexamethasone therapy did not improve outcome. Convulsions may be the initial presentation in some arboviral CNS infections, and status epilepticus is a complication in some cases; appropriate cardiorespiratory support, correction of metabolic disorders, and anticonvulsant therapy with lorazepam should be instituted promptly.
PREVENTION
Vaccines licensed in the United States or Europe are available for three arboviral infections: yellow fever, JE, and TBE. Attenuated yellow fever vaccine and inactivated JE vaccine (produced in Japan) are licensed and commercially available in the United States for use by travelers. Inactivated vaccines for TBE are produced in Europe and are used widely in some countries. Because the risk of acquiring TBE is low for most travelers, vaccination is not recommended routinely, and the vaccine is not available in the United States. Vaccines to prevent hemorrhagic fever with renal syndrome and Kyasanur Forest disease are licensed in Asia. Vaccine development is under way for WNE, dengue, and Ebola hemorrhagic fever, although not necessarily
for travelers’ use. Inactivated, canarypox-vectored and noted DNA equine vaccines for WNE are available commercially.
for travelers’ use. Inactivated, canarypox-vectored and noted DNA equine vaccines for WNE are available commercially.
Vector-borne infections can be prevented by avoiding at-risk locations during transmission seasons or at high-risk times of the day (e.g., avoiding outdoor activity during the period when mosquito vectors are active). Use of protective clothing and repellents can reduce arthropod exposure and bites. Repellents containing picaridin (also known as icaridin or Autan® internationally) and DEET are the most effective formulations; oil of lemon eucalyptus may be as effective as low concentrations of DEET but it should not be used in children <3 years. N, N-diethyltoluamide (DEET)-containing repellents have been used widely since the 1960s, but picaridin, a designer molecule based on DEET, has equal or better repellent activity. In high concentrations, DEET can irritate the skin and produce deep ulcerations. Cases of encephalopathy, including fatal cases, have been reported in children from ages 17 months to 8 years after exposures to formulations containing as little as 10% DEET. Although percutaneous exposure was prolonged in most cases, small quantities and limited exposure have been associated with encephalopathy.
Permethrin [0.5%, Permanone (Louiston International Corp., Easton, PA)] sprayed on clothing and bed nets effectively repels and kills mosquitoes and ticks, and impregnated material remains effective even after several washings. Permethrin shampoo [1%, Nix (Warner Lambert, Morris Plains, NJ)] and permethrin cream [5%, Elimite (Allergen, Inc., Research Triangle Park, NC)] are approved for use on children older than 2 years of age to treat head lice and scabies, and permethrin has been used topically in powders and soaps to repel or kill body lice and other insects. Presumably, these preparations could be used on skin as protection against mosquitoes and ticks. Citronyl, derived from citronella oil, may be more effective than DEET in repelling certain sandflies.
TABLE 184.3. PRECAUTIONS WHEN USING INSECT REPELLENTS | |
|---|---|
|
Other simple measures that reduce exposure to vector mosquitoes include covering exposed areas with clothing, avoiding outdoor activities at dusk and dawn when certain vectors are most active, and using mosquito bed nets and insecticidal room sprays. Ordinary insect nets and screens do not have a mesh sufficiently fine to exclude sandflies, which require finer meshes or permethrin-impregnated nets or clothing. When traveling in tick-infested areas, frequent inspection to remove adherent ticks from clothing, gear, and the body (including the scalp) is recommended.
From a public health perspective, arboviral diseases can be prevented or controlled by combining several approaches, including the elimination or reduction of vector mosquito–breeding sources, periodic application of larvicides or adulticides to reduce mosquito populations, and the emergency application of adulticides to lower vector populations in the face of an epidemic. Specific strategies are tailored to the breeding habitat and habits of individual vectors (Table 184.4).
Several arboviruses (e.g., WNE and St. Louis encephalitis) are transmitted to human populations only after an extensive period of amplification in natural reservoirs (e.g., birds). Surveying transmission in mosquitoes and birds helps to anticipate the likelihood of epidemic transmission occurring, allowing emergency vector control measures to be instituted at an early stage.
Certain arboviral and related zoonotic diseases of public health significance have been grouped below according to the major syndromes with which these agents generally are associated, although overlapping syndromes are common findings.
VIRAL HEMORRHAGIC FEVERS
Viral hemorrhagic fever (VHF) is a loosely defined category that includes infections from a number of viruses causing similar clinical syndromes and sharing a similar severity of disease. However, these viruses are dissimilar in many ways, including their taxonomy, reservoir hosts, and, thus, geographic distributions. Risk factors for exposure also vary among the infections, and specific control methods are required. Although the signs and symptoms of individual VHFs may overlap, their pathogenesis may not; thus, therapeutic approaches may differ as well.
Yellow Fever
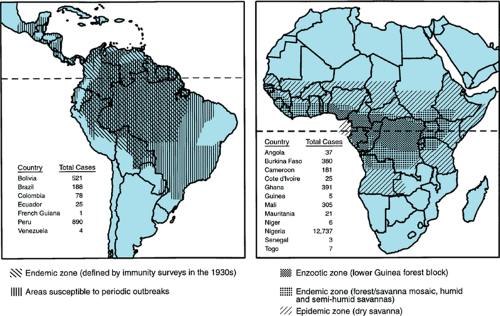
Historically, yellow fever has been feared as one of the great plagues because of its potential to produce thousands of epidemic deaths and the ease of its spread through the movement of viremic humans and infected mosquitoes. In the United States, as recently as 1905, an outbreak in New Orleans produced 5,000 cases and 1,000 fatalities. Now, the transmission of yellow fever is confined to an endemic zone in tropical Africa between 15 degrees north and 10 degrees south and in parts of Central and South America between 10 degrees north and 40 degrees south (Fig. 184.2). The flavivirus is transmitted in a sylvatic or jungle cycle between forest mosquitoes and monkeys, leading to sporadic cases in tangentially infected humans. However, when the virus is introduced to urban locations infested by peridomestic Ae. aegypti, epidemic mosquito-borne human-to-human transmission can result.
In South America, the virus is transmitted in wandering epizootics among monkeys in basins of the Amazon, Orinoco, Catatumbo, Atrato, Magdalena, and other river systems. Treehole Haemagogus and Sabethes mosquitoes are the primary vectors. Approximately 100 to 200 human infections are
reported annually, from January to May, chiefly among pioneers, soldiers, and forestry and agricultural workers whose occupational activities take them into the jungle. Consequently, most cases occur in males between the ages of 15 and 40. Recent cases at the fringe of or introduced into major cities in Brazil and Bolivia have created a threat of epidemic urban yellow fever, prompting the establishment of mass immunization campaigns.
reported annually, from January to May, chiefly among pioneers, soldiers, and forestry and agricultural workers whose occupational activities take them into the jungle. Consequently, most cases occur in males between the ages of 15 and 40. Recent cases at the fringe of or introduced into major cities in Brazil and Bolivia have created a threat of epidemic urban yellow fever, prompting the establishment of mass immunization campaigns.
TABLE 184.4. EXAMPLES OF CONTROL STRATEGIES TO REDUCE TRANSMISSION OF ARBOVIRAL AND ZOONOTIC DISEASES | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In the moist savanna of West and Central Africa, sylvatic mosquito vectors actively transmit the virus among monkeys and humans during the wet season, resulting in an endemic pattern of transmission, with cases frequently occurring in susceptible (unvaccinated) children. Where water storage is practiced and in urban locations, vector Ae. aegypti mosquitoes may be prevalent, holding a potential for epidemic transmission when the virus is introduced. The risk of epidemic transmission is associated with the presence of vectors in 5% or more of households.
Classically, the disease has been divided into three stages of infection, remission, and intoxication. After an incubation period of 3 to 10 days, fever, headache, malaise, and musculoskeletal pain occur suddenly, often accompanied by nausea
and vomiting. Few physical signs are present except conjunctivitis, flushing of the skin, and Faget sign, a relative bradycardia. In most cases, the fever resolves after a few days. However, in approximately 10% of cases, the remission is temporary, lasting only hours to a few days, whereupon illness resumes with the reappearance of toxicity, fever, vomiting, abdominal pain, jaundice, hematemesis, and other hemorrhagic manifestations. Typically, patients are jaundiced, dehydrated, hypotensive, and often oliguric; proteinuria is a characteristic feature and may serve as a helpful differential feature. Myocarditis may complicate the illness. Azotemia, encephalopathy, progressive liver damage, and bleeding lead to death in 30% to 50% of cases.
and vomiting. Few physical signs are present except conjunctivitis, flushing of the skin, and Faget sign, a relative bradycardia. In most cases, the fever resolves after a few days. However, in approximately 10% of cases, the remission is temporary, lasting only hours to a few days, whereupon illness resumes with the reappearance of toxicity, fever, vomiting, abdominal pain, jaundice, hematemesis, and other hemorrhagic manifestations. Typically, patients are jaundiced, dehydrated, hypotensive, and often oliguric; proteinuria is a characteristic feature and may serve as a helpful differential feature. Myocarditis may complicate the illness. Azotemia, encephalopathy, progressive liver damage, and bleeding lead to death in 30% to 50% of cases.
Clinical laboratory examination discloses leukopenia, elevated liver enzymes, and abnormalities of coagulation, including abnormal prothrombin, partial thromboplastin, and clotting times, with significant reductions of liver-dependent clotting factors. Evidence of disseminated intravascular coagulation (DIC) has been reported in some patients.
Numerous mucosal, serosal, and subcutaneous hemorrhages are seen at autopsy. The liver shows a pattern of wide-spread parenchymal necrosis in a characteristic midzonal distribution and microvesicular fatty accumulation, with a minimal mononuclear inflammatory infiltrate. Hepatocytes undergo hyaline degeneration to an apoptotic death, represented by their evolution to eosinophlic-staining Councilman bodies, often found in the canaliculi. The renal medullae are congested and swollen, and the convoluted tubules are damaged. Evidence of myocarditis also may be present.
A diagnosis cannot be made reliably on clinical features alone, because other viral hemorrhagic fevers, viral hepatitis, leptospirosis, malaria, typhus, typhoid fever, and some intoxicants produce a similar clinical presentation. A specific laboratory diagnosis can be made rapidly by identifying viral antigen or genomic sequences in blood or liver biopsy tissue. Serologic diagnosis by detecting specific IgM through enzyme-linked immunosorbent assay (ELISA) in serum is more readily available and also can provide a rapid diagnosis; however, cross reactions from other flaviviral antibodies can limit specificity. The virus can be isolated from blood or tissues in mosquito cell cultures or in suckling mice.
No specific therapy is available, but supportive therapy for hepatic, renal, and circulatory failure may be life-saving. Attention should be given to the prevention and treatment of secondary bacterial infection.
The attenuated yellow fever vaccine made from the 17D virus strain is highly efficacious and safe and probably confers lifelong immunity after a single subcutaneous dose. As a live vaccine, it should not be given to pregnant and immunocompromised persons. The vaccine is contraindicated in infants younger than age 4 months because of a high incidence of vaccine-associated encephalitis. Rare cases also have been reported in adult vaccinees. A potentially fatal multisystemic illness of hepatitis, vascular instability, renal failure, and bleeding diathesis compatible with wild-type yellow fever recently has been recognized as being potentially vaccine-associated, with a rate of 1 per million doses. Advanced age and a genetic component have been suggested as risk factors. The rarity of these events and the potential hazards of the disease (six fatal cases in nonimmunized travelers have been reported since 1996) have not prompted a change in vaccine recommendations, except to reconfirm that the traveler’s destination should be an at-risk location (two of the serious vaccine-associated illnesses were in persons vaccinated for travel to nonendemic areas). In some instances, proof of immunization may be required under international travel regulations. Routine childhood vaccination in endemic areas has been recommended under the World Health Organization (WHO) Expanded Program for Immunization programs, but implementation has been fragmentary. The elimination of mosquito breeding sites of Ae. aegypti is a prerequisite for reducing the risk of epidemic transmission in areas of high human population densities.
New World Arenaviral Infections
Junin, Machupo, Guanarito, and Sabiá viruses, the etiologic agents, respectively, of Argentine (AHF), Bolivian (BHF), Venezuelan (VHF), and Sabiá-associated hemorrhagic fevers, are the only agents among 13 New World arenaviruses that cause human disease in nature, although Flexal and Pichinde viruses have been reported to cause a simple febrile illness after occupational laboratory exposures. These viruses are maintained in specific sigmodontine rodent reservoir hosts. The reservoirs for Junin, Machupo, and Guanarito viruses are Calomys musculinus, Calomys callosus, and Zygodontomys brevicauda, respectively; the reservoir of Sabiá virus has not yet been determined. The pattern of diseases caused by these viruses is enzootic, with intermittent periods of hyperendemic–epidemic transmission. Humans are infected directly from the rodent reservoir through infectious droplets of rodent excreta or by ingestion or direct contact with such infectious material.
AHF and BHF occur in relatively localized geographic foci—AHF chiefly in the agricultural pampas of Buenos Aires and Santa Fe provinces and BHF in the tropical savanna of northeastern Bolivia. VHF has been recognized only in the tropical grassy plains of Portuguesa and adjacent Barinas states in northwestern Venezuela, but the extent of its geographic distribution has not been studied systematically. AHF is almost exclusively an occupational disease of agricultural workers, who become infected during the harvest season from February to May. The adult population has been vaccinated progressively, and infections now are reported increasingly among children. Fetal infection and death have occurred commonly in pregnant women infected with Junin virus, with an associated increase in maternal mortality rates. Junin virus has been isolated from breast milk. BHF and VHF are acquired in a peridomestic setting, with cases occurring in both genders and all age groups. A review of all BHF cases from 1959 through 1962 established that the attack rate for children younger than 10 years of age (4%) was one-third of that for those older than 15 years and that the highest case-fatality ratio was noted in persons younger than 5 years (47%) and older than 55 years (50%). Sabiá virus exposure has caused illness in one person from São Paulo state, Brazil, and in two laboratory workers after occupational exposure.
The clinical features of these diseases are similar. An incubation period of 5 to 19 days is followed by gradual onset of fever, malaise, headache, and myalgia. Retroorbital pain, photophobia, and epigastric abdominal pain may occur; pharyngitis and respiratory symptoms are uncommon occurrences. During the first week, illness is characterized by a flushed appearance, conjunctival injection, generalized lymphadenopathy, and a fine petechial eruption distributed over the skin of the upper trunk, axillae, and the oropharynx. Leukopenia, thrombocytopenia, and proteinuria are characteristic laboratory findings.
A convalescent phase usually occurs after the first week of illness, after which more than one-third of patients develop either neurologic abnormalities or hypotension and hemorrhage associated with a capillary leak syndrome. Overall mortality rates range between 15% to 30%. Progression to neurologic or hemorrhagic syndromes is a strain-specific manifestation of tropic properties of the infecting virus.
The hypotensive-hemorrhagic phase is manifested by gastrointestinal and mucosal bleeding, petechiae, and hemoconcentration with an increasing hematocrit. Mental status changes, ataxia, and tremor are common symptoms but, even when severe, can be accompanied by a normal CSF. Acute renal failure and secondary bacterial infection are additional complications.
Early illness due to the New World arenaviruses cannot be differentiated clinically from other acute infections. Hemorrhagic manifestations may be confused with meningococcemia, leptospirosis, yellow fever, dengue hemorrhagic fever, and idiopathic thrombocytopenia, all of which can occur in the same geographic areas where AHF, BHF, and VHF are found. Establishing a definitive diagnosis requires identification of the virus, viral antigen, or RNA from blood or tissue or demonstration of a specific antibody response by IF, ELISA, or neutralization assays on appropriately timed serum specimens.
Specific therapy for AHF using specific immune plasma reduced mortality rates to less than 1% when 2 units were administered within 8 days of onset of illness. A complication of immune serum therapy is a late-onset neurologic syndrome with fever, ataxia, and tremor occurring 4 to 6 weeks later in 10% of treated patients. Most patients with this late neurologic syndrome recovered fully. Intravenous ribavirin should be considered if specific immune plasma is unavailable or if a patient with AHF presents later than 8 days after onset of disease. Patients must be monitored and treated promptly for shock, hemorrhage, and secondary infection.
Patient care and specimen handling should be performed using standard precautions. The special isolation of patients is not required. Human-to-human transmission has been reported but rarely occurs. An experimental vaccine for AHF (i.e., Candid-1) was more than 95% effective in a large-scale human trial and may be effective for BHF. This vaccine is undergoing phase II trials in children between ages 1 and 14 years. Although control of rodents has reduced outbreaks of BHF in towns, the rural distribution and wider geographic areas where AHF occurs limit the usefulness of rodent control for the latter disease.
Lassa Fever
Initially described in a series of nosocomial epidemics as a lethal hemorrhagic fever associated with a 50% mortality rate, Lassa fever now is known to be a relatively common, usually self-limited, febrile illness in West Africa. Nonetheless, Lassa fever causes as many as 300,000 cases and 5,000 deaths there each year and is a leading cause of fetal and maternal deaths. Lassa fever in children is a well-described syndrome and may account for as many as 10% of febrile illnesses in children admitted to hospitals in endemic areas.
Lassa virus is the only African arenavirus, of which there are five, that produces human disease. The virus is carried persistently by Mastomys huberti and Mastomys erythroleucus, the rodent reservoirs whose infectious excretions are the source of human infections in peridomestic and bush settings.
In adults and children, early illness includes fever, malaise, headache, and musculoskeletal pain. The nonspecific syndrome progresses over the course of 4 to 5 days to include painful pharyngitis, cough, chest pain, and abdominal complaints, including cramping pain, diarrhea, and vomiting. Patients appear ill, are weak, and can be hypotensive. In endemic areas, a purulent pharyngitis, with conjunctivitis, head and neck edema, and mucosal bleeding are highly specific signs of Lassa fever. Chest examination may reveal crepitant rales and evidence of pleural or pericardial effusions. Permanent sensorineural hearing loss can be a late sequela.
Pregnant women are at high risk for having spontaneous abortions and maternal deaths.
Near-term in utero infection appears to be invariably fatal for the newborn infant, and infected mothers have transmitted Lassa virus to nursing infants. Infants with Lassa fever can be severely ill with diarrhea, abdominal distention, and bleeding. The illness can be complicated by pneumonia or seizures and, in children older than 2 years, the mortality rate is 14%. One study in Liberia found a 27% mortality rate among children; of four reported deaths, three were infants with swollen-baby syndrome. This is a highly characteristic syndrome of Lassa fever among children younger than 2 years and is comprised of anasarca, abdominal distention, and bleeding.
Lassa fever typically resolves gradually over the course of 8 to 10 days. In severe cases, the illness may be complicated by hypovolemic shock, encephalopathy, and respiratory distress caused by laryngeal edema, pleural effusions, or pneumonitis. Gingival, gastrointestinal, and vaginal bleeding seem to be caused by circulating inhibitors of hemostasis, as well as platelet and endothelial dysfunction; the bleeding does not reflect DIC.
Patients can have an elevated hematocrit due to hemoconcentration. Leukopenia and proteinuria may be present. A poor prognosis is associated with elevated aspartate aminotransferase levels (150 U/mL) and high levels of circulating virus (≥103.6/μL).
Hepatocellular necrosis, indistinguishable from lesions associated with Marburg, Ebola, and yellow fever viral infections, is the most conspicuous pathologic finding. Splenic and adrenocortical necrosis, interstitial nephritis and pneumonia, and myocarditis also can be found on postmortem examination. High concentrations of virus are found in the absence of distinct pathologic lesions in some organs, including the placenta.
Death from Lassa fever is caused by circulatory collapse related to intravascular volume depletion caused by the dysfunction of capillary endothelium. Shock generally is not attributable to hemorrhage alone.
Rapid recognition of the disease is essential to allow prompt therapy to be initiated. Specific therapy for Lassa fever using intravenous ribavirin is more effective if administered within 6 days of onset of disease and is indicated for adults with markers of poor prognosis. Although the relevance of these prognostic markers in children is undefined, and ribavirin given over prolonged periods in high doses to rats has produced growth retardation, administering intravenous ribavirin to seriously ill children is reasonable, provided the risks and benefits have been explained to the parents.
Lassa virus infection can be diagnosed through viral isolation or the identification of viral antigen or genomic sequences amplified by PCR from blood. Specific IgM antibody against Lassa virus can be detected by ELISA or IF late in acute infections. Neutralizing antibodies may not be demonstrable immediately in recovered patients, and the immunologic mechanisms associated with recovery are undefined.
Supportive care should include the maintenance of fluid and electrolyte balance and respiratory support.
Lassa virus has been transmitted from person to person during hospitalization. All health care should be performed using standard precautions, with the addition of contact and droplet precautions when caring for patients with suspected or confirmed Lassa fever.
Eliminating peridomestic rodents, utilizing rodent-proof storage containers to prevent food contamination, minimizing the handling of trapped rodents, and ensuring thorough cooking of rodents consumed as food are the mainstays of prevention of Lassa fever.
Congo-Crimean Hemorrhagic Fever
CCHF, caused by a nairovirus in the family Bunyaviridae, is a potentially fatal illness transmitted by ticks and by contact with infectious body fluids. The virus reservoirs are Hyalomma ticks, widely distributed in Africa, the Mediterranean, eastern Russia, the Middle East, and western Asia. A broad range of animal species, including domestic livestock, are hosts for the ticks and can serve as both viral amplifiers and vectors. Human infections occur from bites by infectious ticks, from contact with blood of infected animals (e.g., during slaughter), and, in the health care setting, from contact with the body fluids of infected patients. Asymptomatic or mild infections occur, as evidenced by an antibody prevalence of 2% to 30% in farmers and shepherds in endemic areas. The incidence of CCHF is seasonal and follows the rise in tick density during spring and summer.
The incubation period for CCHF is from 2 to 9 days. Illness onset is abrupt and nonspecific, with fever, chills, rigors, intense headache, and generalized muscle aches. Early in the course of illness, the patient can experience chest pain, nausea, vomiting, or diarrhea, with hypotension, conjunctivitis, and tender hepatomegaly noted on examination. Onset of hemorrhagic symptoms occurs after 3 to 6 days of illness, with epistaxis, petechial and often dramatic ecchymotic bleeding into the skin, and upper and lower gastrointestinal tract bleeding. Endothelial dysfunction and capillary leakage are responsible for pulmonary edema and circulatory failure, leading to death in as many as 30% of cases.
Laboratory findings include lymphopenia, thrombocytopenia, and elevated bilirubin and transaminase levels. DIC occurs at the end-stage of illness, whereas early hemorrhagic symptoms are attributed to thrombocytopenia and endothelial and platelet dysfunction, among other factors yet to be described.
Virus RNA and viral antigens can be detected in blood, liver tissue, or autopsy materials obtained during acute infection. Later in the course of infection, specific IgM or IgG can be detected in serum specimens by IF assay, reverse passive hemagglutination inhibition assay, ELISA, or neutralization assays.
A pathologic examination typically reveals disseminated hemorrhages and widespread edema. The liver is involved extensively, with parenchymal necrosis.
Survival is correlated with an effective humoral immune response. Passive immunotherapy with immune plasma can be lifesaving if given early enough. Intravenous ribavirin shows therapeutic promise and might be useful for postexposure prophylaxis. Early suspicion of CCHF based on a clinical presentation and an appropriate epidemiologic history is important to allow consideration of these therapeutic interventions. Secondary bacterial infections are common findings, and they should be anticipated and treated appropriately. The administration of fluids (with careful attention to volume status and pulmonary function) and inotropic agents is indicated to treat circulatory failure.
Travelers to endemic areas should be aware of regional, seasonal, and occupational risk factors. Appropriate measures should be used to prevent tick bites; contact with livestock or freshly slaughtered carcasses should be avoided.
All health care should be provided using standard precautions. Patients with suspected or confirmed CCHF should be cared for by personnel using added droplet and contact precautions. Occupational transmission to health care personnel and secondary transmission to their family members has been documented. A mouse brain-derived vaccine has been developed, but data regarding its efficacy and side-effect profile are very limited. It is not available in the United States.
Filoviral Hemorrhagic Fevers
The hemorrhagic fevers associated with Marburg and Ebola viruses command attention because of their fulminant nature and lethality. Case-fatality during outbreaks are high, with 26% mortality rates among the 37 reported cases of Marburg disease and 53% (Sudan strain) to 88% (Zaire strain) among patients with Ebola hemorrhagic fever (EHF). Based on the size of the virion, its tubular morphology, and distinctive physicochemical properties, these viruses have been grouped in a family of RNA viruses, the Filoviridae. Four subtypes of Ebola virus (Reston, Sudan, Zaire, and Côte d’Ivoire) can be differentiated by genotype and by antigenic variation. The Sudan subtype of Ebola virus is less virulent in animals and is associated with a somewhat lower case-fatality rate in humans than is the Zaire subtype. A single human infection with the Côte d’Ivoire subtype has been confirmed by isolation of the virus. Reston virus was discovered in 1989, as the cause of a highly fatal epizootic hemorrhagic disease of monkeys imported from the Philippines. Despite explosive transmission, with possible airborne spread among colonies, only four humans had serologic evidence of infection; none was symptomatic, suggesting that the Reston subtype may have limited pathogenicity for humans.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree