Anterior Lumbar Interbody Fusion, Disc Replacement, and Corpectomy
D. Alex Stroh
Brad W. Moatz
P. Justin Tortolani
DEFINITION
Lumbar disc degeneration is an age-related process heralded by a loss of disc height and gradual changes to the biochemical structure and biomechanical behavior of the intervertebral disc.
Disc degeneration is not painful in most individuals, but in some patients, the degenerative changes do become painful and lead to the clinical entity known as degenerative disc disease (DDD). It is unclear why disc degeneration is painful in some but not in most.
The etiology of DDD is multifactorial, including genetic and environmental determinants.
Discogenic pain is the term used to describe pain due to a degenerative disc.
ANATOMY
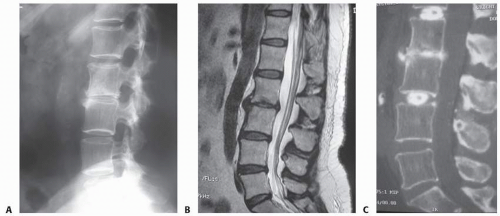
The intervertebral disc is composed of the outer annulus fibrosus and the inner nucleus pulposus (FIG 1A).
The vertebral endplate is composed of cancellous bone in the center and strong, dense, cortical bone along the periphery.
Magnetic resonance imaging (MRI) provides information about the extent of hydration within the disc nucleus. The degenerated disc nucleus will have low signal characteristics (appear dark) on T2-weighted MRI images (FIG 1B).
Dark discs on MRI do not necessarily correlate with symptomatic low back pain.2
Lumbar vertebral bodies are ovoid shaped with a rounded circumference on axial cross-section, except for L5 which is more triangular with a slightly wider base posteriorly bordering the central canal and flattened sides leading to an anterior apex. Care should be taken with transbody screw placement at this level.
PATHOGENESIS
Various mechanisms have been proposed to explain disc degeneration with age:
Reduced nutrition and waste transport
Decreased concentration of viable cells
Loss of matrix proteins, proteoglycans, and water
Degradative enzyme activity
Fatigue failure of the matrix
Herniated nucleus pulposus
Subclinical, indolent disc space infection
Alterations to the vertebral endplate microenvironment such as venous pooling and reduced oxygen tension are additional factors.
Nicotine has known detrimental effects on the intervertebral disc, perhaps via these mechanisms.
Several factors have been implicated in the generation of discogenic pain: altered disc structure and function, release of inflammatory cytokines, and nerve ingrowth into degenerated discs, which under normal conditions are only minimally innervated in the outermost portion of the annulus.
NATURAL HISTORY
Radiographic findings of disc degeneration typically appear around age 30 years.
Posttraumatic disc herniations, vertebral endplate injuries, and genetic factors may predispose patients to earlier presentation.
As structural changes occur within the intervertebral disc, associated changes in the vertebral body endplate become apparent:
Anterior, lateral, or posterior osteophyte formation
Schmorl nodes and cystic cavities along the endplate can be visualized.
Endplate sclerosis
The degenerative changes at the level of the disc, bony endplate, and ultimately the posterior facet joint complex restrict motion at the affected level or levels. At this stage, patients will typically complain more of back stiffness and soreness rather than pain. Neurogenic claudication due to narrowing of the spinal canal and spinal stenosis typically becomes more limiting than complaints of back pain.
The final stage in the natural history of disc degeneration is autofusion.
Patients should be counseled that disc degeneration itself is an inevitable process of aging and that any back pain experienced could, but may not necessarily, be associated with the disc degeneration.
The overwhelming majority of patients have only occasional episodes of low back pain. Long-term disability resulting from DDD is rare.
PATIENT HISTORY AND PHYSICAL FINDINGS
No pathognomonic history or physical examination findings exist for the diagnosis of lumbar DDD.
Discogenic back pain is typically worst in situations in which an axial load is applied to the lumbar spine, as in prolonged sitting or standing with a forward-bent posture (ie, washing dishes, vacuuming, shaving, or brushing teeth).
Conversely, positions such as side-lying (ie, the fetal position) or floating erect in water place the least amount of strain across the intervertebral disc and should therefore provide some pain relief.
Leg pain (in the absence of neural compression), if present, is nonradicular and “referred” in that it does not follow lumbar dermatomes into the lower leg and is not typically associated with loss of motor power, reflex changes, numbness, or tingling.
Patients will occasionally describe a discrete traumatic disc injury in which they first experienced back pain. Imaging studies that depict an old endplate fracture above or below a degenerative disc help corroborate this history.
Loss of truncal musculature fitness from abdominal wall hernias, obesity, and prior abdominal wall surgery (ie, rectus muscle transfer procedures) may worsen discogenic back pain.
Other causes of back pain should be sought in the history, physical examination, and imaging studies, including muscular strain, spondylolysis or spondylolisthesis, herniated nucleus pulposus, compression fracture, pseudarthrosis, tumor, and discitis.
Patients with isolated DDD by definition should have a normal neurologic examination.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Standing plain radiographs
Lateral radiographs allow for measurement of the intervertebral disc height and allow comparison to other lumbar intervertebral discs (FIG 2A).
Anteroposterior (AP) radiographs allow for determination of asymmetric, coronal plain disc degeneration, which may be a precursor to lumbar degenerative scoliosis.
Flexion-extension radiographs may be helpful in diagnosing an occult spondylolisthesis or spondylolysis.
MRI provides excellent visualization of the discs, the degree to which they have degenerated, and the relationship of the discs to the adjacent endplate and surrounding neurologic structures (FIG 2B).
“Modic changes” characterize the endplate on MRI:
Type 0: no changes
Type 1: dark on T1 and bright on T2 (represent marrow edema and inflammation)
Type 2: bright on T1 and isointense/bright on T2 (represent conversion from normal red into yellow marrow).
Type 3: dark on T1 and T2 (subchondral sclerosis)
Modic changes do not always follow DDD but are rare in healthy individuals. They may represent a shifting biomechanical stress distribution across the endplate.13
Provocative discography attempts to reproduce the patient’s typical back pain by pressurizing the disc with normal saline. The patient needs to be awake to provide subjective feedback as to the quality and intensity of the pain. Architectural changes to the disc are inferred by contrast administered with the saline.
Studies have shown that provocative discography leads to accelerated disc degeneration.5
Computed tomography (CT) discography provides more detailed information about the disc morphology after contrast administration (FIG 2C).
Normal laboratory tests, including complete blood count, erythrocyte sedimentation rate, and C-reactive protein, can help rule out a disc space infection; severe disc degeneration can sometimes mimic infection radiologically.
DIFFERENTIAL DIAGNOSIS
DDD
Discitis
Pyogenic vertebral osteomyelitis
NONOPERATIVE MANAGEMENT
DDD is analogous to hip and knee osteoarthritis in that the intervening cartilage (in the case of the disc: collagen, water, and proteoglycans) fails under compressive loads.
Weight reduction and activity modification (avoidance of exacerbating activities) may be effective first-line treatments.
Nonsteroidal anti-inflammatory medications
Acupuncture or massage therapy
Physical therapy with aquatic or dry land exercises
Gentle pelvic traction
Methylprednisolone (Solu Medrol) taper
Epidural injections
Narcotic medications for severe episodes of pain
SURGICAL MANAGEMENT
Indications
Discogenic back pain refractory to nonoperative management
Discitis with pyogenic vertebral osteomyelitis refractory to nonoperative management
Spinal deformity requiring radical discectomy
Revision cases for pseudarthrosis
A thorough and complete discectomy improves the effectiveness of anterior interbody fusion by creating a wide surface area of exposed bone.
Interbody reconstruction and fusion can be accomplished by a variety of methods, including structural autogenous bone graft (iliac crest or fibula), structural allograft (ie, femoral or humeral ring, femoral head, machined bone dowel), or synthetic device (titanium, polyetheretherketone [PEEK], carbon fiber, composite) packed with cancellous bone or collagen sponges impregnated with bone morphogenic protein 2 (BMP-2).
Lumbar total disc replacement (LTDR) was initially adopted in Europe for preservation of segmental motion and avoidance of adjacent-level disc disease; however, prospective randomized investigational device exemption (IDE) trials in the United States failed to reveal superiority of LTDR when compared to anterior fusion. This information plus the rare but catastrophic complications associated with LTDR migrations have precluded widespread use in the United States.
Regardless of the method used, prerequisites are that the interbody spacer be strong enough to resist intervertebral compressive loads and provide an appropriate biologic environment for healing.
Preoperative Planning
Plain radiographs, MRI, or CT scans should be carefully evaluated for undiagnosed spondylolysis or spondylolisthesis, which may alter the surgical plan.
Templates can be used with plain radiographs or MRI scans to gauge the size of the final implant to be used.
Oversized implants can lead to undesired stretch on neurologic structures and reduced motion of lumbar disc replacements.
Screw trajectories in stand-alone anterior lumbar interbody fusion (ALIF) devices must be templated to avoid violation of the central canal or neuroforamina.
The level of the confluence of the common iliac veins into the inferior vena cava and the bifurcation of the aorta can be located on the axial MRI scans.
At L5-S1, the pubic symphysis occasionally precludes appropriate visualization and instrumentation of the disc space in patients with a deep-seated L5-S1 relative to the pelvis. Evaluation of the lateral radiograph with the pubis on the film is critical to visualize the trajectory into the disc space and avoid this miscalculation.
Positioning
See Chapter 49.
The patient is placed over an inflatable pillow over a 1-inch thick foam pad, which is placed on the mattress of the operating table. The pillow allows for modulation of lordosis throughout the procedure and the foam pad props the patient up, allowing the arms to be tucked posteriorly out of the plane of the spine during imaging.
Positioning over the break in the table allows for increased lordosis if needed.
The use of fluoroscopic C-arm imaging is crucial for appropriate patient and implant positioning. It is helpful to verify that adequate fluoroscopic imaging of operative landmarks can be achieved after the patient is positioned but before the incision is made.
Approach
See Chapter 49 on Anterior Lumbar Approach.
Anterior retroperitoneal approaches will typically allow access to the lumbar discs from L2 to L3 to the sacrum.
The renal vessels limit more proximal extension of the exposure.
Lateral exposures to the lumbar spine are required for access to the L2 vertebra and above.
TECHNIQUES
▪ Anterior Lumbar Radical Discectomy
Exposure
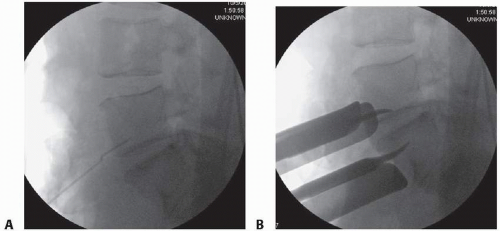
Identify the intervertebral disc and mark the midline with a spinal needle or screw placed into the vertebral body (we prefer not to place a needle into the disc space because this may create unwanted disc injury) (TECH FIG 1A).
Use AP and lateral fluoroscopic imaging to check the midline. The midline marker also serves to verify the spinal level.
At L5-S1, retract the left common iliac artery and vein to the patient’s left and the right common iliac artery and vein to the right. At levels above L5-S1, the aorta and inferior vena cava must be mobilized to the patient’s right.
The great vessels can be held in their retracted position using handheld Hohmann retractors, custom-designed pins, or Kwires, all of which can be advanced directly into the vertebral bodies (virtually eliminating the risk of vessel migration into the field of interest) (TECH FIG 1B).
Alternatively, stainless steel vein retractors or radiolucent retractors can be fixed to the arms of an abdominal retractor system (Omni) or floating, Endo ring-type retractor system. These blade retractors have the disadvantage of allowing vessel migration into the field by sliding under the retractor blades as motion occurs during the procedure. The advantage of the radiolucent retractors is that better visualization of the operative field is possible with fluoroscopy. In addition, blade-type retractors can be easily manipulated during the procedure without having to reinsert into the vertebral body.
Attempt to retract the vessels as far lateral as you can to allow for the widest possible view of the intervertebral disc. Poor visualization at this stage will compromise the quality of the discectomy and any ensuing interbody device placement.
Removing the Disc
Using a no. 10 blade on a long handle, incise the intervertebral disc starting laterally along the superior endplate and move toward midline. Always move away from the vessels to avoid an accidental lateral plunge into the great vessels. The blade should be inserted between the cartilage endplate and bone if possible, and we use both hands on the knife shank for optimal control and coordination (TECH FIG 2A,B).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree