CHAPTER 21 Anatomic Single-Bundle Anterior Cruciate Ligament Reconstruction
Anterior cruciate ligament (ACL) reconstruction has evolved from the open techniques of the 1970s and early 1980s to current arthroscopic techniques. During the course of that evolution, the endoscopic transtibial reconstructive technique became popular.1 With this technique, the position of the femoral tunnel is dictated by the direction and position of the tibial tunnel. Unfortunately, not enough attention was paid to this fact and through the 1990s, more transtibial endoscopic ACL reconstructions were done in a very sagittal alignment, resulting in persistent rotatory instability, even if the graft remained intact. Howell and colleagues2,3 were among the first to recognize this as a clinical problem. They noted that with such an alignment of the graft, one of two problems would ensue, stretching of the graft as it wrapped about the posterior cruciate ligament (PCL) in flexion or failure to regain flexion. They described the coronal angle as the angle in the frontal plane between the axis of the joint line and axis of the tibial tunnel. For coronal angles more than 75 degrees (more vertical), these problems of stretching of the graft or failure to regain motion were encountered. For coronal angles less than 75 degrees, optimal results were obtained.
Raffo and associates4 have recently described a reproducible anatomic landmark for the starting point of the tibial tunnel on the anteromedial tibia as the point of intersection between the anterior edge of the medial collateral ligament and superior border of the gracilis tendon, as a point that will reproducibly yield a coronal angle of 70 degrees or less. This technique for establishing the tibial tunnel start point is my preference for bone-patellar tendon-bone (B-PT-B) grafts. This allows creation of a femoral socket that will accommodate 30 mm of bone block in the anatomic attachment site of the ACL. Because an interference screw can be placed proximal to the bone block within the socket, the graft can be well positioned near the center of the ACL footprint on the femur. Others have described a similar starting point for the tibial tunnel, but have relied on techniques to identify it5 that are less reproducible than those I have used.
Fu and coworkers6 were instrumental in recognizing the issues with the sagittally placed ACL reconstruction, and have exquisitely defined the anatomy of the ACL—in particular, its two-bundle configuration with separate and distinct sites of origin from the femur and insertion onto the tibia. They demonstrated that both anatomically placed double- or single-bundle reconstructions were superior biomechanically and kinematically to nonanatomic techniques.7,8 Gardiner and coworkers have recently shown in the cadaver laboratory that the anatomic single- bundle technique is kinematically equal to the double-bundle technique.9 With soft tissue grafts, placing the graft into the center of the footprint of the ACL on the lateral wall of the intercondylar notch and obtaining rigid fixation may best be done through a medial portal technique. This has become my routine for soft tissue grafts. Confirmation of use of the anatomically placed single-bundle reconstructive technique has recently come from a meta-analysis by Meredic and colleagues,10 which showed no advantage of double-bundle techniques over the anatomic single-bundle procedure.
Graft choice is another variable that may affect ACL outcome. A number of meta-analyses comparing autograft patellar tendon to autograft hamstring tendons have demonstrated that there are distinct differences between the outcomes.11,12 The patellar tendon graft in these analyses has consistently yielded a higher percentage of stable knees, with a greater percentage of athletes returning to preinjury activities, when compared with the soft tissue alternatives. It is apparent that patellar tendon grafts are not without issues. Anterior knee pain and quadriceps weakness have been identified as problems.13 These make hamstring grafts an attractive alternative for the patient with less demanding work or athletic pursuits.. Finally, allografts have become more frequently used for ACL reconstruction. In a younger and more active patient, these may lead to significantly greater rates of failure—two or three times greater in a recent meta-analysis—when compared with autograft alternatives.14,15 All these data considered together have resulted in a graft selection algorithm that I routinely discuss with patients when considering ACL reconstruction (Table 21-1). Autologous B-PT-B is preferred for the high-demand competitive athlete. An autologous four-strand hamstring graft is selected for the moderately demanding recreational athlete, with less time of exposure to high-risk activities. Finally, allograft tissue is reserved forspecial cases, which include age older than 40 years, autologous tendon issues such as failed autograft, or prior tendon injury. Each of these graft choices must be placed in an anatomic location on both the femur and tibia and rigidly fixed to result in a stable knee postsurgery that will allow individuals to pursue their work or recreational activities.
TABLE 21-1 Anterior Cruciate Ligament Graft Selection Algorithm
| Patient Characteristics | ACL Graft Choice |
|---|---|
| Competitive athlete: high school, college, professional | B-PT-B autograft |
| Recreational athlete <40 yr | Quadrupled hamstring autograft |
| Recreational athlete >40 yr; mitigating factors (e.g., revision) | Allograft—B-PT-B or tibialis anterior |
TREATMENT
Arthroscopic Technique: Anatomic Soft Tissue Anterior Cruciate Ligament Reconstruction
Preparation of the graft is another important step. For either graft, the overall length is determined by the height of the patient. I routinely double a 21-cm graft (yielding a 10.5-cm construct) for patients 5’8” tall or shorter. For patients between 5’8” and 6’2”, I use a 22-cm graft doubled over to yield an 11-cm construct. Finally, for patients taller than 6’2”, I use a 23-cm graft doubled over to yield an 11.5-cm overall construct length. These lengths are important, because tensioning the graft on the tibial side with the Intrafix and its tensioner is most easily accomplished with 5 to 10 mm of graft extending from the tibial tunnel when the Intrafix sheath and screw are placed. For the tibialis grafts, I split each end in half for a length of 7 cm to yield four ends for tensioning. I whipstitch with a baseball-type suture configuration using a no. 2 high-strength suture, for a length of 5 cm. The central 5 cm of the graft is whipped together by looping a no. 2 high-strength tie around the graft. This facilitates passage of the sheath for the femoral Intrafix, with its triangular flange between the two bundles. The suture material also improves the fixation properties of the construct, reducing potential slippage of the graft past the device with cyclic loading (Fig. 21-1).
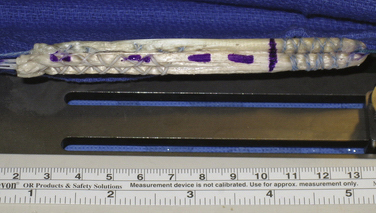
FIGURE 21-1 Soft tissue graft prepared for use with Femoral and Tibial Intrafix devices (DePuy Mitek).
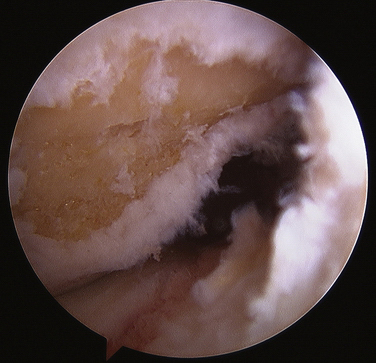
Minimal notch preparation is needed for this technique. In a chronic situation, any osteophytes that might potentially impinge on the graft are removed. If the anterior aspect of the notch is narrow, notchplasty to expand this and reduce the likelihood of impingement on the graft is also appropriate. I prefer to preserve the ACL footprint intact on the femur (Video 1), clear it centrally prior to guide pin passage, and mark my preferred guide pin starting point with a curette (Fig. 21-2) (Video 2). Use of an over the back guide of the femoral aimer with an offset to preserve 1.5 to 2 mm of bone posterior to the femoral socket will yield excellent anatomic positioning of the graft.
Prior to placement of the femoral guide pin, it is essential that an adequate amount of the fat pad be resected so that at 120 degrees of flexion, the fat pad does not obstruct vision of the notch (Video 3). Once this is done, the over the back guide is introduced through the medial portal. The medial portal needs to be low (just above the medial meniscus) and medial so that the over the back femoral guide is touching the medial femoral condyle when the guide pin is passed (Fig. 21-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree