Allograft Transplantation for Articular Defects of the Knee
Petros J. Boscainos
Catherine F. Kellett
Allan E. Gross
INDICATIONS/CONTRAINDICATIONS
The use of fresh osteochondral allografts for osteochondral defects of the knee is based on a scientific rationale and on long-term clinical experience.
Fresh avascular osteochondral allografts, if harvested within 24 hours of death and preserved at 4° C, show 100% viability of cartilage at 4 days (7,12,33). Although freezing decreases the immunogenicity of the bone to some degree, it also results in decreased chondrocyte viability (13,38). Even cryopreservation and controlled rates of freezing and thawing will not achieve an acceptable degree of cartilage viability (13). The matrix that surrounds the chondrocytes isolates them from the host’s immune cells and prevents host sensitization (23). The avascular bone remains structurally intact and mechanically strong until it is replaced by host bone by creeping substitution (30). Although the osteocytes will not survive unless the graft is vascularized, the use of immunosuppressive drugs to counter the increase in immunogenicity, that this would produce, cannot be justified in this setting. In a series of failed fresh osteoarticular allografts between 12 and 84 months after transplantation, none has demonstrated histologic evidence of transplant rejection (21). Long-term chondrocyte viability of fresh osteochondral allografts has been confirmed in a number of studies, even at 17 years after transplantation (9,11,26,30).
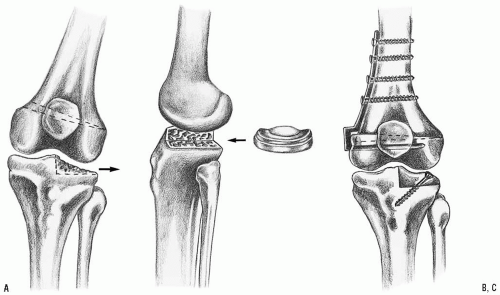
Based on that scientific rationale, cadaveric allografts with viable cartilage and avascular bone, which provides an intact structure until host bone replaces it by creeping substitution, provide a reconstructive solution for young, high-demand patients where implants or an arthrodesis is not desirable (Fig. 32-1) (17,36). Osteochondral allografts provide flexibility in terms of the size of defect that can be reconstructed. Both femoral and tibial defects can be addressed and where needed, the allograft meniscus can also be transplanted (17).
Appropriate patient selection is paramount to satisfactory outcome. A number of patient characteristics have been identified that are predictive of success.
Diagnosis
McDermott et al (25) reviewed the first 100 patients who received fresh, small-fragment osteochondral allografts for articular defects in and around the knee. Initially, these grafts were performed for unicompartmental
osteoarthritis, spontaneous osteonecrosis of the knee, steroid-induced avascular necrosis of the femoral condyles, osteochondritis dissecans, and most commonly, traumatic defects. Grafts performed for trauma had the best results (10). Those done for primary osteoarthritis had poor results. Meyers and coworkers also had poor results with fresh grafts placed into osteoarthritic knees (27). Garret (15) produced excellent midterm results in treating patients with osteochondritis dissecans.
osteoarthritis, spontaneous osteonecrosis of the knee, steroid-induced avascular necrosis of the femoral condyles, osteochondritis dissecans, and most commonly, traumatic defects. Grafts performed for trauma had the best results (10). Those done for primary osteoarthritis had poor results. Meyers and coworkers also had poor results with fresh grafts placed into osteoarthritic knees (27). Garret (15) produced excellent midterm results in treating patients with osteochondritis dissecans.
It may be concluded that posttraumatic defects and osteochondritis dissecans of the knee are the best indications for fresh grafting (6,8,9,15,17,25,27,30,41).
It has been our experience that the best results with fresh osteochondral allografts are in patients with unipolar posttraumatic defects and osteochondritis dissecans of the knee. At our institution osteochondral allografts are no longer used for treating osteoarthritis, spontaneous osteonecrosis, or steroid induced osteonecrosis, or in patients with inflammatory arthropathy. If the posttraumatic defect has been present for long enough to cause severe degenerative changes in the opposing articular surface, the graft is contraindicated. Also the patient must be compliant and capable of rehabilitation.
Age
Beaver et al (3), using Kaplan-Meier survivorship analysis, demonstrated that patients younger than 60 years of age with posttraumatic defects had better results with osteochondral allografts than patients older than 60 years. Fortunately, the great majority of posttrauma patients are in their second and third decades.
Site
Size
Recent advances in other techniques for cartilage repair and resurfacing, such as microfracture technique, autologous chondrocyte transplantation, osteochondral autografts, and periosteal grafts (6,
24,28,29,31,37), have reduced the role of allograft transplantation to defects larger than 3 cm in diameter and 1 cm deep (20).
24,28,29,31,37), have reduced the role of allograft transplantation to defects larger than 3 cm in diameter and 1 cm deep (20).
Deformity
Most osteoarticular defects have a concomitant malalignment of the limb. In order to unload the compartment that has received the transplant, this malalignment should be addressed with a realignment osteotomy of the tibia or femur, as appropriate (18,19). For this purpose, a lateral-closing wedge high tibial osteotomy is performed for valgus realignment and a medial-closing wedge, distal femoral osteotomy is used to realign the limb into varus (20) (Fig. 32-2). Lately, the senior author has been using a medial-opening wedge high tibial osteotomy for valgus realignment and fixation with a Puddu plate (32) (Fig. 32-3). If indicated, the osteotomy should be performed at the same time as the osteochondral graft transplantation. Delayed osteotomy could be reserved as a salvage procedure for a deteriorating graft when the mechanical axis passes through the grafted compartment in very young patients (20).
PREOPERATIVE PLANNING
The preoperative assessment includes a clinical and radiographic evaluation of the injured knee. The presence of previous scars or hardware that needs to be taken out might determine the configuration of the skin incision.
Routine views of the knee and a 3-foot standing radiograph will provide the data necessary to estimate the location, size, and structure of the defect and required graft. A careful assessment for the presence of degenerative changes and of the need to correct alignment must be done. The biomechanical axis is used to evaluate deformity and plan correction. Computed tomography (CT) scans and magnetic resonance imaging (MRI) are not required routinely but, if available, can help define the defect.
In some patients, previous arthroscopic data might help evaluate the size of the defect, secondary degenerative changes, and status of the opposing surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree