Fig. 4.1
Coronal MRI showing significant disruption to the posterolateral corner structures (This image was published in Miller et al. [6], with permission)
MRI scans are obtained on almost all patients to provide more information and further clarify as to what to expect during surgery (Fig. 4.2). They are especially useful if a ligament or tendon is avulsed without a large enough bony fragment to be seen on X-ray as this can direct your dissection intraoperatively. All of the above physical exam tests are important, as an MRI can misrepresent injuries and do not elucidate the clinical relevance of a “high-grade tear” or of “ligament attenuation.” MRI images should be used in conjunction with and not in lieu of a physical exam.
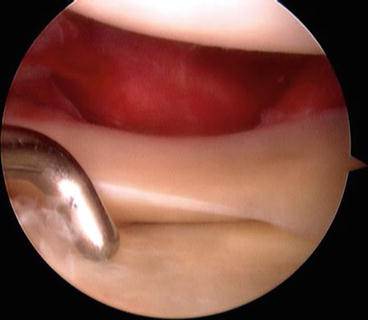

Fig. 4.2
Arthroscopic view of a lateral capsular avulsion off of the femur (i.e., the meniscus remains with the tibia) as well as a positive drive-through sign
Once the patient and clinician have decided on surgical management, it is of our opinion that acutely managing these injuries results in superior outcomes, and this has been validated in multiple studies [8, 9]. We usually define as acute as surgically addressing the injury within 2 weeks.
4.2 Surgical Technique
In order to access the knee posterolateral structures, it is important to have the appropriate patient positioning and OR table setup. The patient is positioned supine on a radiolucent OR table with a bump under the ipsilateral buttock. Another option includes a lazy lateral position with a bean bag positioner. If the PCL is being reconstructed concomitantly with an inlay technique, then a lateral decubitus position is used. An AFO-type leg holder and nonsterile tourniquet are placed on the operative extremity.
Each case includes an arthroscopic evaluation of the joint to fully characterize the intra-articular pathology. A drive-through sign (>1 cm of joint space opening) is often present in the lateral compartment, and it should be noted if the meniscus remains close to the tibia or femur as this will give the surgeon an idea from which side the joint capsule may be avulsed and, therefore, which side needs to be repaired [10] (Fig. 4.3). Meniscal tears are easily seen as the wide joint space allows uncommon visualization of the entire lateral meniscus. These are usually repaired using an inside-out or open technique utilizing the open lateral incision which is soon to be developed. Prior to the initial diagnostic arthroscopy, we always perform an initial dissection of the lateral knee in order to allow for egress of the arthroscopic fluid. This help to greatly reduce the risk for iatrogenic leg compartment syndrome.
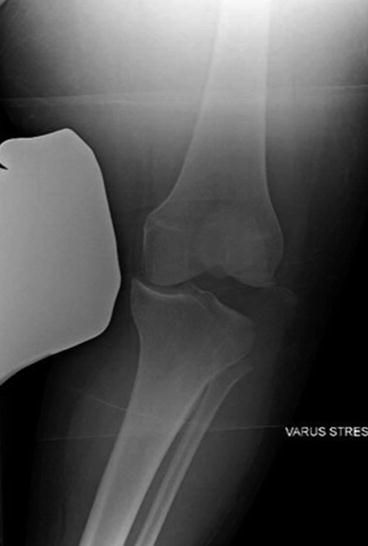

Fig. 4.3
AP radiograph with a varus stress applied with significant lateral joint space opening with displacement of the avulsed fibular head (i.e., arcuate sign)
After drawing out the lateral and posterolateral landmarks (fibular head, Gerdy’s tubercle, tibial tubercle, posterior edge of the iliotibial (IT) band), an 8–10 cm vertical incision is centered at the joint line just anterior to the fibula. The planned incision is drawn out in extension and is made with the knee in 90° of flexion. The subcutaneous tissue is dissected to expose the IT band and the biceps tendon. At this point, it is important to identify and protect the peroneal nerve which is located just posterior to the biceps. In order to have safe access to the proximal fibula during the reconstruction, you must carefully dissect out the nerve around the neck of the fibula. The nerve is protected throughout the remainder of the case. Next, the interval between the iliotibial tract (posterior 1/3 of the iliotibial band) and the biceps is developed to allow identification of the lateral collateral ligament, popliteofibular ligament, and popliteus tendon. A second interval (slightly anterior and distal) is found between the iliotibial band and iliotibial tract where the lateral femoral condyle can be accessed. The second interval allows visualization of the popliteus and LCL. Using this approach, the peroneal nerve is easily retracted posteriorly and out of the surgical field. Alternative approaches have been described such as making multiple deep windows or intervals to access particular structures. We have found that a larger single incision with two adequately sized intervals provides better access to the deep structures and allows the surgeon to continuously keep an eye on the peroneal nerve.
When acutely addressing these injuries (ideally within 2 weeks), individual structures are able to be identified before the whole area becomes encased in scar tissue. These ligaments and tendons should be dissected out to see if they are amenable to repair, to help locate accurate insertions and origins for graft placement, and to provide local native ligament to augment reconstructions. Although repair is often possible in these acute situations, it is sometimes very difficult to achieve secure fixation as the damaged tissue has been stretched to failure, and thus the ends are often frayed and do not hold suture well. One exception to that rule is seen with body avulsion injuries as seen radiographically as the arcuate sign (Fig. 4.3). This type of failure is particularly amenable to anatomic repair with suture anchors or, depending on the bony fragment size, screw and washer constructs. If secured and immobilized appropriately postoperatively, avulsion injuries reliably heal because of the strong bone to bone reparative process. It is important to remove all soft tissue intervening between the bony fragments and to not entrap the peroneal nerve during fixation around the fibular head. Additionally, lateral meniscal tears are commonly seen in these injuries and should be repaired when possible although techniques for this procedure will not be discussed in this chapter. Primary repair of mid-substance injuries of the LCL with sutures, or reattachment of soft tissue avulsions with suture anchors, heal less dependably due to the watershed nature of this region, the quality of the damaged tissue, and less secure fixation methods. An acute repair of the LCL in an end-to-end fashion augmented with a strip of the long head of the biceps has been described, and Coobs et al. demonstrated that reconstruction of the LCL has been shown to be biomechanically superior to repair if no augmentation is added [11, 12]. It is for these reasons that reconstruction is the preferred technique even in acute injuries. In acute PLC injuries, Stannard et al. had a 37 % failure rate when repaired compared to 9 % when reconstructed using the modified 2-tail technique [13]. In our experience with these situations, more reliable immediate and long-term stability is achieved by using a soft tissue graft to reconstruct the torn structure than by repairing.
There are many techniques which have been described to reconstruct the posterolateral corner. They are often divided into anatomic and nonanatomic, with nonanatomic being mainly of historical interest. Commonly used techniques include the Muller popliteal bypass procedure, Larson figure of eight PLC reconstruction, two-tail PLC reconstruction, three-tail PLC reconstruction, and LaPrade PLC reconstruction. There are small variations between these procedures, but all rely on free soft tissue grafts and various fixation methods (interference screws, staples, screw and washers) to reconstruct native anatomy. At our institution, we mostly employ combined Larson figure of eight and Muller popliteal bypass techniques, and these are described below.
The Larson figure of eight is used to reconstruct the popliteofibular ligament (PFL) and the LCL. As previously mentioned, the injured ligamentous structures in this area often have very frayed ends and are not amenable to direct repair so allograft or autograft tissue is used. Available donor tissue include the hamstrings (HS), tibialis anterior (TA), Achilles, or quadriceps; however, our preferred graft options are autograft hamstring including the semitendinosus and gracilis. Often, these autografts are needed for cruciate ligament reconstruction so semitendinosus allograft is most commonly selected. The larger diameter of the graft should be applied to the Muller popliteal bypass. For the Larson figure of eight, a length of at least 22–24 cm is required to allow for adequate fixation. The grafts are prepared on the back table using a #2 Fiberwire (Arthrex, Naples, Florida); however, any similarly robust suture placed in a whip-stitch technique would suffice.
While the graft is being prepared, the fibular tunnel for the Larson reconstruction is created. An anterior distal to posterior proximal tunnel is made through the thickest part of the fibular head, being very careful to retract the peroneal nerve out of the path of the drill bit. The tunnel is initially located with a guide wire over which we use a cannulated drill bit to finalize the preparation of our fibular tunnel. The tunnel sizes are chosen based on a sizing guide which measures the graft diameter. For semitendinosus and tibialis anterior, most tunnel sizes are 5 or 6 mm. For gracilis, the tunnel size is normally 4 or 4.5 mm.
The Muller popliteal bypass is then performed to reestablish the rotational control provided by the disrupted popliteus tendon. Using a second guide wire, we localize our popliteus tunnel in the tibia with the intended trajectory being from Gerdy’s tubercle to 1 cm medial and 1 cm inferior from the posterolateral aspect joint line of the tibia, i.e., the musculotendinous junction of the popliteus. Prior to placing this guide wire, the lateral head of the gastrocnemius and popliteus muscle is bluntly dissected off of the posterior tibia and retracted to protect the neurovascular structures. This is over-drilled with an appropriate-sized reamer. Next, the femoral insertion point for both grafts is located at the midpoint between the LCL insertion at the lateral epicondyle (which is usually palpable through the IT band) and the popliteus tendon insertion. A guide wire is placed to mark this location, but we do not yet drill this tunnel. Fluoroscopy is used to check the pin placement. One graft is then fed into the fibular tunnel with equal length kept on both sides. The two free ends of this graft are twisted and looped around the wire on lateral femoral epicondyle. The limb running from the anterior fibula to posterior to the guide wire re-creates the LCL. The second graft is fed through the tibia tunnel, around the posterolateral joint line, and looped around the wire as well. It is best to bring this graft initially anteriorly around the wire and then looping it counterclockwise. We use an 18-gauge Luque wire to facilitate this passage of the grafts through the tunnels. Additionally, we ensure that the graft limbs are deep to the IT band and biceps. At this point, the knee is taken through a full range of motion to assess for uniform graft tension as well as any restraints to flexion or extension. It is important that the wire is located at the isometric point as to not overtighten the joint and cause range of motion limitations postoperatively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








