Fig. 9.1
Palpation of a left Achilles tendon. The tendon should be squeezed between the thumb and the index fingers; this procedure should be performed for all the length of the tendon to appreciate any swelling or nodule
Other tests are performed with the patient standing. Tendon stretching during passive dorsiflexion of the ankle is performed placing the affected leg backward and leaning forward; the heel must not lift off the ground. The test is positive if the patient complains of pain during the exercise. Other tests are the single-leg heel raise, performed by the patient rising up onto tiptoes and lowering back to the floor on the affected leg, and the hop test, performed by the patient hopping forward over a line marked on the floor. Weakness and/or pain during these exercises are suspected for Achilles tendinopathy.
Anyway, in evaluating reproducibility and accuracy of all these clinical test for Achilles tendinopathy, Hutchison et al. demonstrate that “palpation” and “self-reported pain” are the most valid ones [19].
Imaging studies are important to confirm the diagnosis and to evaluate the entity of the disease and its evolution. They comprise magnetic resonance imaging, ultrasonography, color and power Doppler, and sonoelastography.
Magnetic resonance imaging (MRI) provides extensive information about the internal and external morphology of tendon. It is useful to evaluate the stages of chronic degeneration and to differentiate between paratendinopathy and tendinopathy. Mucoid degeneration (altered collagen fiber structure and increased extracellular matrix) is visible as increased signal intensity areas on T1- and T2-weighted images (Fig. 9.2). Any peritendinous fluid or inflammation is clearly visible as hypointense in T1 and hyperintense in T2 [20].
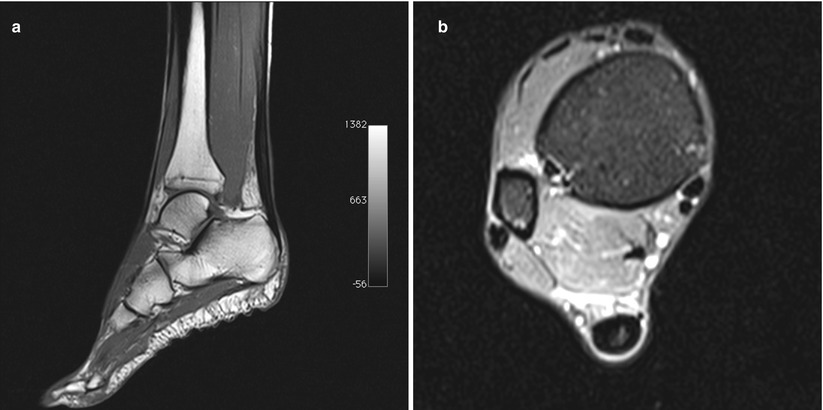

Fig. 9.2
Magnetic resonance imaging of a 38-year-old female runner with left midportion Achilles tendinopathy. (a) T1 sagittal view shows midportion tendon thickening with an area of increased signal. (b) Proton density axial view shows a hyperintense area into the tendon, sign of intratendinous mucoid degeneration
Ultrasonography is more widespread and easier to obtain than MRI, even if it is operator dependent. Tendon thickening and peritendinous swelling or edema are easily detectable with US. Intratendinous hypoechoic areas are signs of Achilles tendinosis. One of the main advantages of US in comparison with other imaging modalities is the interactive facility, which helps in reproducing symptoms by transducer compression and concentrate on the pathologic area [21].
Color and power Doppler improve standard ultrasound tendon imaging demonstrating entity and pattern of the blood flow in the tissue. In normal Achilles tendon, blood flow is not detectable; in Achilles tendon disorders, blood flow increases and is linked with greater pain scores, poorer function, and longer symptoms in the Achilles tendon [22].
Sonoelastography is a novel US technique that can assess the elastic properties of tissues [23]. After a mechanical compression (performed by the examiner with the probe), any tissue is deformed with a specific spatial and temporal pattern. The elastic properties of normal Achilles tendon are altered under pathological conditions, and a distinct intratendinous softening can be detected by sonoelastography. This new promising technique could be useful in detecting early-stage tendinopathy and in monitoring its evolution during treatments.
9.4 Treatment Strategy
Treatment differs according to the site of the disorder: insertional or non-insertional.
9.4.1 Non-insertional Achilles Tendinopathy
9.4.1.1 Nonoperative Treatment
Conservative treatment is recommended as the initial strategy by most authors [24].
Identification and correction of possible etiological factors combined with a symptom-related approach permit to return to previous activities in most patients; an 8-year follow-up study showed a failure rate of only 29 % with conservative treatment [25].
Initial rest (complete or modified activity), modification of training regimes, specific exercises, and correction of underlying lower limb malalignment with orthoses (heel lift, change of shoes, corrections of malalignments) are the mainstay of the treatment [26, 27].
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) is debatable because there is no evidence of their effects on symptoms; moreover, the histologic absence of inflammatory cells in the tendinopathic tissue led its use questionable [28, 29]. NSAIDs should even have detrimental effects; an in vitro study demonstrated that celecoxib inhibits tendon cell migration and proliferation [30].
Corticosteroid injections are reported to reduce pain and swelling. These early benefits are counterbalanced by reports of adverse effects in up to 82 % of corticosteroid trials [31], in particular tendon rupture [32, 33] and decreased tendon strength [34, 35].
Eccentric exercises are beneficial in the early treatment of non-insertional Achilles tendinopathy; they lead to normalization of tendon structure and reduction in neovascularization, even if the exact mechanism by which they work is poorly understood [36, 37].
Alfredson et al. reported return to normal activities at 12 weeks in 82 % of cases in the group treated with eccentric exercises, compared with 36 % of patients treated with concentric exercises used concentric exercises; improvement was still present at 12 months [38, 39]. Anyway, the results of eccentric exercises observed from other study groups out from Scandinavia are less convincing, with only a 50–60 % of good outcome [40, 41].
The results of extracorporeal shock wave therapy in treating midportion Achilles tendinopathy are conflicting. Low-energy shock wave therapy and eccentric training produced comparable results, superior to the wait-and-see policy [41]. The combination of these two treatment strategies led to significant improvement and better outcome than eccentric exercises alone [42].
Platelet-rich plasma (PRP) injection is going to become widely used in several orthopedic areas. It demonstrated improved tendon healing compared with control in vitro, but a randomized double-blind placebo-controlled trial showed no difference in improvement in pain and activity at 6 months [43, 44].
Sclerosing therapy with polidocanol injections has the objective of destroying neovessels and the consequent neonerves in the paratenon to reduce pain and even leading intratendinous remodeling. The first authors introducing this treatment strategy reported good clinical results with 38 of 42 patients satisfied at 2-year follow-up [45]. However, newer contrasting results have been reported. In a recent study, van Sterkenburg et al. reported that only 44 % of 53 tendons were painless or minimally painful at 6 weeks after treatment with 3 sessions of ethoxysclerol injections [46]. Moreover, at 2.7–5.1-year follow-up, 53 % of tendons had undergone additional (nonoperative or surgical) treatment.
Peritendinous high-volume injections of anesthetic (10 ml 0.5 % bupivacaine) and normal saline solution (40 ml) seem effective in reducing pain and improving function; anyway, these studies are limited case series and with limited follow-up [47, 48].
Intratendinous hyperosmolar dextrose injections (prolotherapy) are another promising treatment in vitro but wide clinical studies with mid- and long-term follow-up are lacking [49].
9.4.1.2 Surgical Treatment
Conservative treatment for midportion Achilles tendinopathy fails to resolve symptoms and to allow sports resumption in 24–45.5 % of cases [50].
Traditional surgical treatment consists in open release of adhesions with or without resection of the paratenon. Multiple tenotomies are performed to initiate vascular ingrowth from the surrounding tissue, and intratendinous areas of tendinopathy are excised [51–53]. If debridement is superior to 50 % or the tendon width, augmentation should be performed with plantaris tendon or turndown flaps for small defects or tendon transfer (peroneus brevis, flexor digitorum longus, flexor hallucis longus) for larger defects [54, 55].
Recently, the transfer of a soleus pedicle graft into a central tenotomy was proposed, with the aim of increasing tendon blood supply and promoting a faster tendon healing (Fig. 9.3) [56]. When compared with longitudinal tenotomies, despite similar outcomes in postoperative functional scores at the 4-year follow-up, soleus transfer allows a faster recovery and return to run but has a higher incidence of tendon thickening [56].
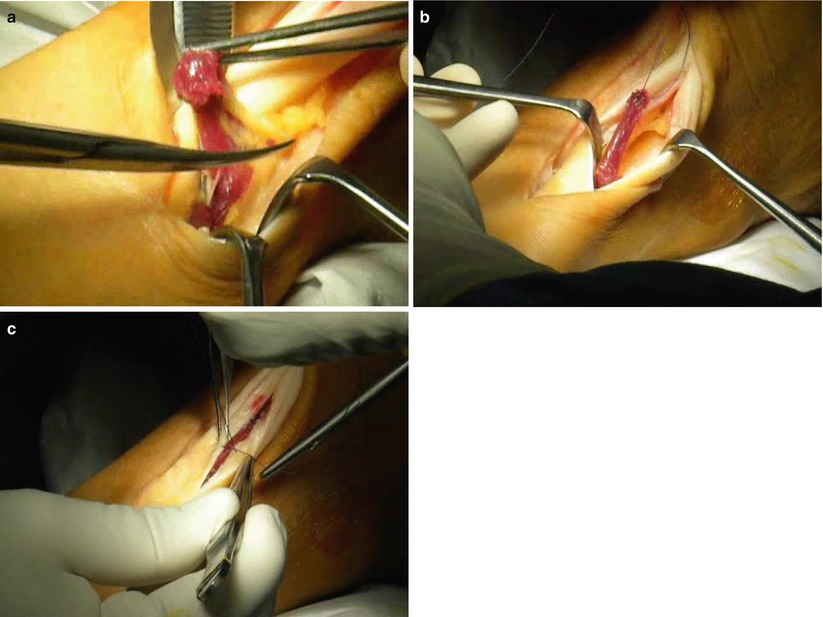

Fig. 9.3
Intraoperatory view of the soleus fiber transfer technique performed in a left Achilles tendon. (a) After a lateral paratendon incision, a cylindrical bundle of the inferolateral portion of the soleus muscle is isolated by blunt dissection. (b) The muscle bundle, left attached at its distal end, is flipped 180° and placed inside a full-thickness tenotomy in a “sandwich” way. (c) The bundle is secured with stitches, and the tenotomy is closed
The main concern about open surgery for midportion Achilles tendinopathy is the non-negligible risk of wound healing complications [53, 55]. Other complications are nerve and soft tissues damage. Paavola et al., in a large series of 432 consecutive patients, reported an overall complication rate of 11 % at 5 months of follow-up [55]; the most frequent ones were wound edge necrosis (3 %), superficial infection (2.5 %), and sural nerve irritation (1 %).
Stripping of the paratenon follows the same principle of sclerosing injection: destroying the neovessel and the neonerves around the Achilles tendon in order to reduce pain. This technique reported good results in 75–100 % of cases [57, 58], even if they were case series with a limited number of patients treated. Moreover, Naidu et al. reported a complication rate of 7 %, mainly due to delayed wound healing [58]. Endoscopic tendon debridement seems promising, but studies with a consistent number of patients are lacking [59, 60]. Maquirriain performed 27 endoscopic paratenon debridement and longitudinal tenotomies in 24 patients with midportion Achilles tendinopathy [59]. All patients had an improved clinical outcome at a mean final follow-up of 7.7 years (range 5–14). 96 % of patients obtained a complete resolution of symptoms. Pearce et al. added plantaris tendon release to tenoscopy in 11 patients with midportion Achilles tendinopathy [60]. After a minimum follow-up of 2 years, the mean AOFAS scores improved from 68 preoperatively to 92 postoperatively (p = 0.0002). Eight patients were satisfied.
9.4.2 Insertional Achilles Tendinopathy
9.4.2.1 Nonoperative Treatment
The first therapeutic strategy is to reduce load-bearing activities and modification of training regimes. Immobilization should be avoided for its detrimental effect on tendon structure and strength [61].
Heel lift permits to reduce pressure on the retrocalcaneal bursa by plantar flexing the heel, potentially favoring healing of the tendon insertion. Orthoses to correct any malalignment are helpful to improve symptoms [62].
The same eccentric exercise program already applied in midportion Achilles tendinopathy was also evaluated in insertional diseases. The good results obtained for non-insertional disease were not replicated for insertional pathology; it showed success rates of 28–32 % [62, 63]. Eccentric exercises with ankle dorsiflexion were supposed to be detrimental in insertional pathology. Jonsson et al. proposed eccentric training using floor-level exercises only, founding improved outcomes in 67 % of cases [64].
Regular stretching may increase the working length of the muscle–tendon unit; indeed, it increases ankle dorsiflexion by only 1° [65].
Shock wave treatment seems effective in treating insertional tendinopathy; Rompe et al. reported good or excellent results after 4 months in almost 83 % of patients compared to 39 % in conventionally treated patients [66].
Corticosteroids are rarely used because of their historic risk of partial or complete tendon rupture [67]. Injections of hyperosmolar dextrose solution, sclerosing agents, or platelet-rich plasma seemed promising in treating insertional pathologies, but only one study for each treatment is reported [68–70].
9.4.2.2 Operative Treatment
Endoscopic calcaneoplasty and bursa debridement showed good results in 75–95 % of patients treated, with small scars, minimal debridement, and rapid recovery [62, 71].
Open surgery is more invasive and restricted for recalcitrant disease; it consists of debridement of the tendon insertion, removal of bursal tissue and of bony prominence, reattachment of the insertion as required (with bone anchors/screws or trans-osseous sutures), and/or augmentation of the tendon with a tendon transfer/graft [62]. Even the 50 % of the tendon insertion can be debrided with minimal risk or rupture [72].
The best graft used for augmentation of the Achilles tendon is the flexor hallucis longus (FHL). It is in proximity, is synergist, and has good vascularity [73]. Peroneus brevis should also be employed, but subsequent ankle instability and foot inversion can occur [74]. Even if weaker than the FHL and more difficult to harvest, the flexor digitorum longus should also be used [75]. Other sources of tendon tissue are autograft as quadriceps, patellar tendon, or hamstring. Also the V–Y advancement of the musculotendinous junction of the gastrocnemius should be performed in repairing insertional defects longer than 2 cm.
9.5 Rehabilitation and Return to Play
Achilles tendinopathies represent a big chapter in sport traumatology and, as previously shown, a broad spectrum of treatments are possible. The choice depends from the type of pathology, its location and degree, and the preference and the level of expertise of the clinician. The number of different treatments reflects the difficulty in treating these pathologies and their often unpredictable outcome.
Eccentric exercises are historically proposed as a 12-week program; it led to satisfactory outcome in 50–60 % of midportion Achilles tendinopathies [40, 41] and in 28–32 % of insertional tendinopathies [62, 63].
In 20–30 % of cases, conservative treatment fails in improving symptoms and allowing sports resumption [75]. In these cases, surgery is the second step of treatment. Minimally invasive surgery permits a lower incidence of complications, in particular wound infection or delayed healing, and also a faster recovery. All procedures that address only peritendinous structures showed a recovery time of 6 weeks to 6 months. After these techniques, complete mobilization of the ankle and partial weight bearing is permitted till the first day after surgery. On the contrary, surgical procedures that address the tendon itself (debridement, incisions, augmentations) temporarily weaken it. Therefore, recovery time can be extended up to 3–18 months. In these cases, the ankle should be immobilized in a dorsal splint or in a cast up to 3 weeks with the ankle in slight plantar flexion. Partial weight bearing will be allowed after cast removal. Mobilization will be performed passively, then actively in open kinetic chain. Once there is complete recovery of the range of motion, closed kinetic chain exercises start. Cycling and walking in the water play an important role in recovery range of motion and strength of calf muscles. When the patient is able to walk without limping, gentle stretching and light jogging are allowed. Exercises for static and dynamic proprioception are central in the rehabilitation process.
Sport-specific exercises can be introduced when the calf muscle strength is close to normality (close to the contralateral side). A close monitoring of the healing process by imaging is suggested; ultrasonography is a fast and easy way to do it, even if it is operator dependent. Magnetic resonance imaging permits a finest evaluation, even if it is not often readily available.
Return to play is permitted when the patient passes specific functional tests as the single-leg hop test, the triple-leg hop test, the shuttle run test, the side jump test, and the carioca test or with the isokinetic evaluation.
References
1.
2.
Khan KM, Cook JL, Kannus P, Maffulli N, Bonar SF (2002) Time to abandon the “tendinitis” myth. BMJ 324:626–627PubMedCentralCrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







