Abdominal Vascular Injury
Brandon H. Tieu
Minhao Zhou
Donald D. Trunkey
Abdominal vascular injuries are highly lethal and a difficult challenge even for the most experienced trauma surgeons.1 Patients can arrive in a stable condition with a contained hematoma, or in extremis and coagulopathic state with uncontrolled hemorrhage.2,3 Hemorrhagic shock continues to be the second leading cause of mortality following trauma, but is the leading cause of preventable death.4 It is important that early surgical control of bleeding is established as resuscitation and correction of coagulopathy are occurring. These injuries are relatively uncommon and are a result of penetrating injury in 88% to 95% of cases and blunt injury in 5% to 10%.5,6,7,8 Accessing the retroperitoneal vessels can be difficult and iatrogenic injury can occur with rapid dissection through hematomas resulting in further blood loss. Owing to the location of the abdominal vasculature, associated injuries are the rule rather than the exception, with an estimated two to four associated intra-abdominal injuries that occur with visceral blood vessel trauma.6,7 The presence of associated injuries increases the difficulty and time of management of abdominal vascular trauma even in damage control procedures. Even after initial control of exsanguinating hemorrhage, patients can still succumb to multisystem organ failure resulting in delayed mortality. Successful management of these injuries requires expedient surgical intervention, thorough knowledge of the anatomy and surgical precision to minimize iatrogenic injuries, and maximize the patient’s chance of survival.
INCIDENCE
Historically, abdominal vascular injuries are more commonly seen in urban trauma settings than in the combat theater. Asensio et al. reported on 302 patients with abdominal vascular injuries over a 72-month period at a busy Level I trauma center that admits 7,000 to 7,500 cases per year.1 A 30-year review of 5,760 cardiovascular injuries from another busy urban trauma center found 1,947 (33.8%) abdominal vascular injuries.9 Goaley et al. reports that the Emory University Trauma service at Grady Memorial Hospital in Atlanta consistently treats 30 or more patients per year with abdominal vascular injuries.5
Compare this to reports during military conflict. In a review of 2,471 arterial injuries from World War II by Debakey and Simeone,10 they found that only 49 (2%) of those were abdominal arterial injuries. Rich et al. reported that of 1,000 arterial injuries from the Vietnam War, only 29 (2.9%) involved the abdominal vessels.11 In a recent publication from Clouse et al. reporting 301 vascular injuries from the Balad Vascular Registry in Iraq, only 18 (6%) were abdominal vascular injuries.12
DIAGNOSIS
Diagnosing abdominal vascular injuries starts with an astute awareness based on the mechanism of trauma that an injury may have occurred. Penetrating injuries in the midline should raise the suspicion for an injury to the aorta, inferior vena cava (IVC), or their respective branches.13
Patient presentation following abdominal vascular injuries is dependent on whether there is active exsanguination or if the bleeding is contained. If the hematoma is contained within the retroperitoneum or base of the mesentery, patients can either be hemodynamically stable or hypotensive but respond to intravenous (IV) fluid
resuscitation. For those who have uncontrolled hemorrhage into the abdomen or retroperitoneum, they will present with severe hypotension or in cardiopulmonary arrest with losses of 40% to 50% of their blood volume.6 The abdominal examination may reveal slight abdominal discomfort or acute peritonitis and a distended and rigid abdomen.
resuscitation. For those who have uncontrolled hemorrhage into the abdomen or retroperitoneum, they will present with severe hypotension or in cardiopulmonary arrest with losses of 40% to 50% of their blood volume.6 The abdominal examination may reveal slight abdominal discomfort or acute peritonitis and a distended and rigid abdomen.
Baseline laboratory values that should be drawn include a complete blood count, an arterial blood gas, and coagulation studies. Rarely will these tests help with the diagnosis of an abdominal vascular injury but repeating them can help monitor resuscitation efforts as interventions are carried out. If the patient is in severe shock and in immediate need of surgical intervention then laboratory investigations should not delay transfer to the operating room (OR).
A plain radiograph of the abdomen can be quickly obtained in the trauma bay and may detect the presence, location, and trajectory of a penetrating missile. A computed tomography (CT) scan may show other associated injuries in a hemodynamically stable patient, but rarely are imaging studies helpful or required for diagnosis of abdominal vascular injury. The focused assessment with sonography for trauma (FAST) examination can be highly sensitive and specific for detecting hemoperitoneum in hypotensive patients following blunt abdominal trauma when performed by trauma team members,14 but its reliability for penetrating trauma has yet to be determined.6
INITIAL RESUSCITATION
All trauma patients should be evaluated according to Advance Trauma Life Support protocols. For those with suspected abdominal vascular injury, after establishing a secure airway, assure adequate venous access with at least two large-bore IV lines, and start volume replacement with lactated Ringers solution. For those who do not respond to the initial 2 L of crystalloid solution, blood transfusions should be initiated. An attempt should be made to maintain a systolic blood pressure of 80 mm Hg until vascular control has been achieved. A Foley catheter should be placed after ruling out urethral injury to monitor resuscitation and test for hematuria, which could be a result of renal injury. Maneuvers to maintain normothermia should be instituted such as warming of the trauma bay, heated blankets, removal of wet clothing, and warmed IV fluids. Hypothermia can augment the development of coagulopathy and along with acidosis can result in the “lethal triad” of hypothermia, acidosis, and coagulopathy. For severely injured patients with coagulopathy, early use of fresh frozen plasma should be considered.15,16
Patients with penetrating injuries to the abdomen and present in extremis may need an emergency department (ED) thoracotomy. These patients present in cardiopulmonary arrest or are in severe shock that is unresponsive to resuscitation.6 ED thoracotomy allows for open cardiopulmonary massage and aortic cross-damping to maintain cerebral and coronary arterial blood flow.17 This technique limits intra-abdominal arterial bleeding but does not affect hemorrhage from venous injuries. Morbidity from thoracotomy itself includes an additional site for bleeding, another body cavity where heat can be lost, ischemia distal to the cross-clamp, and predisposes the patient to reperfusion injuries. Survival after ED thoracotomy range from 0% to 19.6% and for OR thoracotomy up to 10%.1,18,19,20 Most of those patients who live longer than 24 hours after ED thoracotomy should have good neurologic recovery.21
OPERATIVE MANAGEMENT
The patient should have a standard trauma sterile preparation and draping from the neck to the mid thigh
and down to the operating table laterally to cover the entire torso. Assure that adequate amounts of all blood products are available. The OR should be warmed, heating blankets kept on the operating table, and warm air covers placed over the patient’s legs, head, and upper extremities. Warmed fluids should be administered if needed and the temperature of the ventilator cascade increased to 42°C.6 Prophylactic antibiotics should be given before surgery.
and down to the operating table laterally to cover the entire torso. Assure that adequate amounts of all blood products are available. The OR should be warmed, heating blankets kept on the operating table, and warm air covers placed over the patient’s legs, head, and upper extremities. Warmed fluids should be administered if needed and the temperature of the ventilator cascade increased to 42°C.6 Prophylactic antibiotics should be given before surgery.
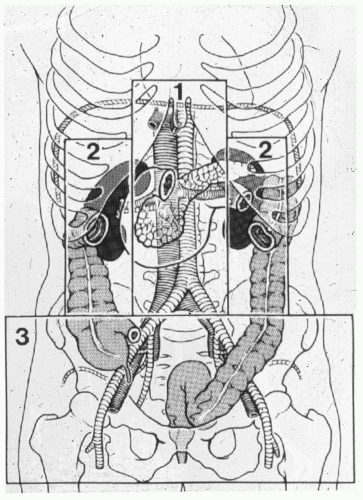
A standard midline incision should be made from the xyphoid process to the pubis. Immediate control of obvious life-threatening hemorrhage should be achieved, followed by control of gross spillage into the abdominal cavity. For solid organ hemorrhage, abdominal packs can be used to control bleeding. Direct digital pressure, sponge sticks, and formal proximal and distal control can be used for vascular hemorrhage. All four quadrants can be quickly packed for continued surface bleeding or if the bleeding cannot be immediately identified before embarking on a thorough inspection of the abdominal cavity and retroperitoneum. Feliciano et al. described a systematic approach to the management of vascular injuries that consists of classifying the location of the retroperitoneal hematoma in zones (see Fig. 1).22
AORTIC INJURIES
The aorta is located in zone I, midline retroperitoneum, and an aortic injury should be suspected in all penetrating trauma to this area. Injuries to the aorta can be divided into either supramesocolic or inframesocolic. Zone I retroperitoneal supramesocolic hematoma or hemorrhage can be an injury to the suprarenal aorta, the celiac axis, the proximal superior mesenteric artery (SMA), or proximal renal artery. Supramesocolic vascular injuries generally carry a higher mortality rate (65% to 90%) than inframesocolic injuries (55% to 66%) due to its difficult anatomic location.1,23,24,25,26 If active hemorrhage is encountered, temporary control can be achieved by packing with laparotomy pads or manually compressing the aorta at the aortic hiatus with your fingers, an aortic root compressor, or placement of an aortic clamp.25,27 Placement of a damp at this location can be difficult and will require some dissection. Rapid exposure of the supraceliac abdominal aorta is achieved by dividing the lesser omentum, retracting the stomach and esophagus to the left, and manually dissecting the area just below the aortic hiatus of the diaphragm.28 The left crus of the diaphragm may require transection to facilitate the
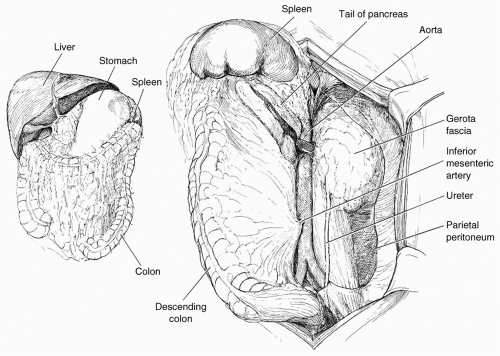
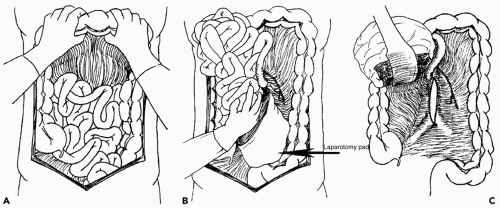
exposure. Control of the suprarenal abdominal aorta is difficult due the anteriorly located celiac axis and SMA. Once hemorrhage is controlled, a left medial visceral rotation can completely expose the abdominal aorta (see Fig. 2). This is performed by incising the avascular line of Toldt of the left colon from the splenocolic flexure to the level of the distal sigmoid colon. The splenorenal ligament is also mobilized with a combination of sharp and blunt dissection. The left-sided viscera including the left colon, spleen, stomach, tail, and body of the pancreas are rotated using blunt dissection medially (Lim maneuver). For better access to the origin of the left renal artery, the left kidney can be mobilized and included in the medial rotation (Mattox maneuver). This exposes the entire length of the aorta including the aortic hiatus, the origin of the celiac axis, the origin of the SMA, the left iliac system, and the origin of the right common iliac artery. If the hemorrhage or hematoma is located in zone I inframesocolic area, rapid exposure can be achieved by retracting the transverse colon and mesocolon cephalad, eviscerating the small bowel to the right, and opening the midline retroperitoneum until the left renal vein is exposed (see Fig. 3). A proximal aortic cross-damp can be placed inferior to the renal vessels to gain proximal control. For distal control, division of the midline retroperitoneum can be continued down to the aortic bifurcation taking care to avoid the origin of the inferior mesenteric artery (IMA) on the left. If the presence of a large retroperitoneal hematoma is distorting the entire inframesocolic area, the vascular defect is most likely located under the highest point of the hematoma (Mt. Everest phenomenon).25 Once proximal and distal vascular control is achieved, surgical repair can proceed. Small injuries to the aorta should be adequately debrided and repaired primarily with 3-0 or 4-0 vascular suture. For larger defects where primary repair will significantly narrow the lumen of the aorta, patch aortoplasty is indicated. For more extensive injuries where a large portion of the aorta needs to be resected, an interposition graft with 12 or 14 mm polytetrafluoroethylene (PTFE) or Darcon graft can be used. The graft is sewn in with 3-0 or 4-0 vascular suture, both ends of the aorta are flushed before the distal anastomosis is completed, and the distal aortic cross-clamp is removed before the final knot is tied to eliminate air from the system. Contamination from perforated viscous of the stomach, small bowel, and/or colon must be controlled and care must be taken to prevent graft infection. Vigorous intraoperative irrigation, proper graft coverage, and appropriate use of perioperative antibiotics can all be employed to decrease the incidence of graft infection, which is fairly rare in otherwise young healthy trauma patients.25 The retroperitoneum should always be used to cover the repair with absorbable sutures in a watertight manner when feasible. Additional maneuvers for tissue coverage of the aortic graft include mobilizing the gastrocolic omentum, placing it into the lesser sac superiorly, and then bringing it down over the infrarenal aorta through a hole in the left transverse mesocolon. The gastrocolic omentum can also be mobilized away from the right side of the colon and brought into the left lateral gutter just below the ligament of Treitz to cover the graft. The omental pedicle is sutured in place to cover the graft and suture line to help prevent aortoenteric fistula.29,30
exposure. Control of the suprarenal abdominal aorta is difficult due the anteriorly located celiac axis and SMA. Once hemorrhage is controlled, a left medial visceral rotation can completely expose the abdominal aorta (see Fig. 2). This is performed by incising the avascular line of Toldt of the left colon from the splenocolic flexure to the level of the distal sigmoid colon. The splenorenal ligament is also mobilized with a combination of sharp and blunt dissection. The left-sided viscera including the left colon, spleen, stomach, tail, and body of the pancreas are rotated using blunt dissection medially (Lim maneuver). For better access to the origin of the left renal artery, the left kidney can be mobilized and included in the medial rotation (Mattox maneuver). This exposes the entire length of the aorta including the aortic hiatus, the origin of the celiac axis, the origin of the SMA, the left iliac system, and the origin of the right common iliac artery. If the hemorrhage or hematoma is located in zone I inframesocolic area, rapid exposure can be achieved by retracting the transverse colon and mesocolon cephalad, eviscerating the small bowel to the right, and opening the midline retroperitoneum until the left renal vein is exposed (see Fig. 3). A proximal aortic cross-damp can be placed inferior to the renal vessels to gain proximal control. For distal control, division of the midline retroperitoneum can be continued down to the aortic bifurcation taking care to avoid the origin of the inferior mesenteric artery (IMA) on the left. If the presence of a large retroperitoneal hematoma is distorting the entire inframesocolic area, the vascular defect is most likely located under the highest point of the hematoma (Mt. Everest phenomenon).25 Once proximal and distal vascular control is achieved, surgical repair can proceed. Small injuries to the aorta should be adequately debrided and repaired primarily with 3-0 or 4-0 vascular suture. For larger defects where primary repair will significantly narrow the lumen of the aorta, patch aortoplasty is indicated. For more extensive injuries where a large portion of the aorta needs to be resected, an interposition graft with 12 or 14 mm polytetrafluoroethylene (PTFE) or Darcon graft can be used. The graft is sewn in with 3-0 or 4-0 vascular suture, both ends of the aorta are flushed before the distal anastomosis is completed, and the distal aortic cross-clamp is removed before the final knot is tied to eliminate air from the system. Contamination from perforated viscous of the stomach, small bowel, and/or colon must be controlled and care must be taken to prevent graft infection. Vigorous intraoperative irrigation, proper graft coverage, and appropriate use of perioperative antibiotics can all be employed to decrease the incidence of graft infection, which is fairly rare in otherwise young healthy trauma patients.25 The retroperitoneum should always be used to cover the repair with absorbable sutures in a watertight manner when feasible. Additional maneuvers for tissue coverage of the aortic graft include mobilizing the gastrocolic omentum, placing it into the lesser sac superiorly, and then bringing it down over the infrarenal aorta through a hole in the left transverse mesocolon. The gastrocolic omentum can also be mobilized away from the right side of the colon and brought into the left lateral gutter just below the ligament of Treitz to cover the graft. The omental pedicle is sutured in place to cover the graft and suture line to help prevent aortoenteric fistula.29,30
Cross-clamping the abdominal aorta in a hemodynamically unstable patient can cause profound ischemia to the lower extremities. The anesthesiologist should be notified when the proximal aortic cross-damp is removed slowly
while fluids and IV bicarbonate are rapidly infused to reverse washout acidosis from the previously ischemic lower extremities. Ischemia and reperfusion injury places the patient at very high risk for lower extremity compartment syndrome. Lower extremity compartment pressures should be measured (>30 mm Hg is high) and bilateral below-the-knee two-incision four-compartment fasciotomies should not be delayed.
while fluids and IV bicarbonate are rapidly infused to reverse washout acidosis from the previously ischemic lower extremities. Ischemia and reperfusion injury places the patient at very high risk for lower extremity compartment syndrome. Lower extremity compartment pressures should be measured (>30 mm Hg is high) and bilateral below-the-knee two-incision four-compartment fasciotomies should not be delayed.
INFERIOR VENA CAVA INJURIES
The vast majority of IVC injuries are due to penetrating trauma, with gunshot wounds being the most prevalent. If the injury is due to blunt trauma, it usually involves the retrohepatic or intrapericardial sections of the IVC due to rapid deceleration and shearing injury. Regardless of the mechanism of injury, IVC trauma carries approximately 50% mortality. The worse prognosis occurs in patients who arrive with hemodynamic instability or injuries involving the suprarenal or retrohepatic vena cava.31,32
The IVC has numerous tributaries including the right gonadal vein, right adrenal vein, renal veins, four to five pairs of lumbar veins, and hepatic and phrenic veins. Owing to this extensive collateral network, it is difficult to obtain complete proximal and distal control of the IVC.31
The venous circulation is a low-pressure system. More than half the number of patients who present will have a contained hematoma.31 In patients with a stable midline nonpulsatile hematoma, controversy surrounds whether this needs to be explored. As many as 40% of patients may die from exsanguination after disruption of the hematoma secondary to surgical exploration.33 Indications for exploration include concurrent injuries to the pancreas, duodenum, kidney, ureter, colon, or an associated arterial injury. Nonpulsatile hematomas due to injuries to the retrohepatic vena cava are probably best left unexplored because of the high risk and low probability of associated retroperitoneal injuries in that area. If active bleeding from the IVC is encountered, initial control can be obtained with manual compression of the bleeding point with a tightly rolled gauze pack until a more definitive exposure and control can be achieved.
Exposure of the suprahepatic IVC can be difficult. Upon entering the abdomen and gaining initial control of hemorrhage with packs, a diagnostic maneuver that can be useful is to gently retract the dome of the right lobe of the liver caudad. If a gush of blood is encountered, this usually implies an injury to the right or left hepatic vein. If there appears to be a large retroperitoneal hematoma when mobilizing the right lobe of the liver, it may become obvious that there is a retrohepatic cava injury. If the surgeon suspects that a hepatic vein has been injured, packs should be replaced and a sternotomy performed. This will allow control of the intrapericardial portion of the IVC with a Rummel tourniquet (Haeney technique).34 This, in combination with the Pringle maneuver, allows the surgeon approximately 30 minutes to repair the injured vessel.
The diaphragm can be split in the midline in a vertical manner to reveal the upper portion of the subdiaphragmatic cava and the hepatic veins. These can be repaired primarily or if one is completely avulsed, an appropriate lobectomy should be carried out. The best approach for blunt injuries to the retrohepatic cava is from the right side with medial rotation of the liver to the midline. This will often expose segmental veins coming from segment one directly into the cava that have been lacerated or the cava has a linear laceration reflecting that these veins have been detached from the anterior surface. For penetrating injuries to the retrohepatic cava and in rare instances it may be necessary to do a left hepatic lobectomy to gain access to the cava. This is preferred to a right hepatic lobectomy because the right lobe constitutes >60% of the liver mass. Intracaval shunts have also been described as well as the venoveno bypass.35 However, we have found that the Haeney technique, as described in the preceding text, is preferable in most instances. Isolation of the liver blood supply using the Haeney technique can only be used for brief periods of time (up to 30 minutes) because the patient is in hypovolemic shock and the liver is already hypoxic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree