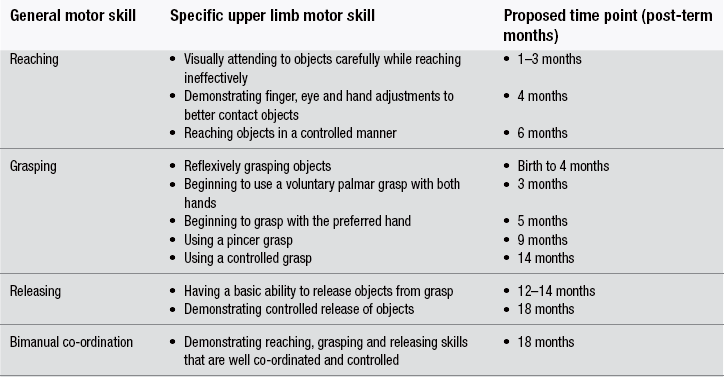
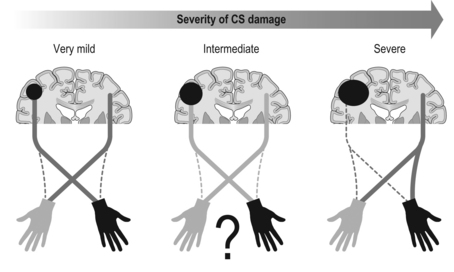
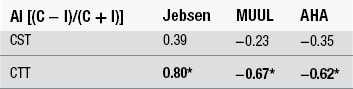
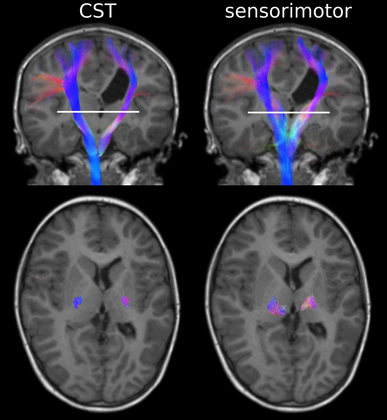
13 Definitive diagnosis of hemiplegia Critical periods of typical upper limb motor development Cortical reorganization after an early brain lesion: a critical window Current evidence for early upper limb interventions for infants with hemiplegia Potential early interventions for infants with asymmetric brain lesions Early bilateral stimulation of reaching and grasping with motor and sensory components Infant modified constraint-induced movement therapy Early action observation training The main focus of early intervention for infants with asymmetric brain lesions who may progress to classification of unilateral cerebral palsy (UCP) is very early and accurate detection of the brain lesion, followed by provision of an enriched environment and training to maximize upper limb function during critical periods of development. The challenge for clinicians and researchers are the limited quantitative tools available to identify the problem and measure progress as well as the paucity of evidence for efficacy of very early upper limb rehabilitation. In this book chapter we will: (1) focus on the current knowledge of critical periods of early upper limb development and the potential neural correlates; (2) summarize the evidence for efficacy of current interventions; and (3) explore new options for early stimulation of the damaged cortex to achieve better symmetry of upper limb motor development. Lessons learned from our clinical trials of intensive upper limb interventions in school-aged children with UCP, including the impact of dose, density and components of training on neuroplasticity, will be discussed in light of the implications for training the young infant with an asymmetric brain lesion in the first two years of life. Infants with early asymmetric brain injury are at high risk of developing congenital hemiplegia as a result of presumed prenatal, perinatal or postnatal brain injury (Cioni et al., 1999). The underlying injuries usually consist of periventricular white matter damage (e.g. periventricular leukomalakia or venous infarctions), cortical and/or deep grey matter damage (e.g. arterial ischaemic stroke) and, less frequently, brain malformations of one hemisphere (e.g. focal cortical dysplasia or unilateral schizencephaly). Congenital hemiplegia is the most common type of cerebral palsy (CP), with a prevalence of 1 in 1300 live births (Wiklund and Uvebrant, 1991). These infants have impaired upper limb motor function and can experience difficulties participating in activities of daily life (e.g. feeding, play and self-care). There are, broadly speaking, two common clinical presentations of asymmetric brain lesions: early or delayed. Early presentation consists of perinatal onset of neurological symptoms, or seizures, or reduced movement at 24 to 48 hours post-birth with verification on cranial ultrasound and/or magnetic resonance imaging (MRI) of the presence of a unilateral or asymmetric brain lesion. Specific imaging protocols may be needed for the diagnosis in the early phases, such as diffusion MRI to identify an acute stroke in the first hours or days (Huppi, 2002). In a delayed presentation, the infant may have an initially uncomplicated perinatal course and may not show signs of stroke or asymmetric brain injury until three to seven months of age when unilateral weakness and early hand preference start to manifest (Golomb et al., 2001; Lynch and Nelson, 2001). The current most predictive tools for early diagnosis of CP are a combination of brain MRI at term and a general movements (GMs) assessment in the fidgety period (Spittle et al., 2009). Specifically, GMs at 1 month and 3 months post-term age are highly associated with white matter abnormalities on MRI at term age (Spittle et al., 2009). The GMs assessment is a well-validated and reliable tool, and is more sensitive at predicting CP than other motor assessments used in infancy (Noble and Boyd, 2011; Spittle et al., 2008). Neuromotor assessments utilized in the neonatal period have strong validity to detect CP in infants born preterm on criterion assessments at 12 months corrected age (such as the Bayley Developmental Scales II and III); moderate evaluative validity (on the Test of Motor Impairment, TIMP), as well as prediction of minor motor difficulties using the GMs (Hadders-Algra et al., 2004; Noble and Boyd, 2011). The classification of early writhing GMs is abnormal although asymmetries are not yet visible, while asymmetry of fidgety GMs around 12 weeks post-term can be the first definitive clinical sign of hemiplegia (Cioni et al., 2000; Guzzetta, 2010; Guzzetta et al., 2003). Very early detection of hemiparesis frequently requires serial evaluation of subtle signs of interlimb differences or asymmetries in muscle resistance to passive movement, muscle stiffness, upper limb reaching (both spontaneous and purposeful), and grasp strength (Heathcock et al., 2008). Both bimanual and unimanual reaching with early strong hand preference at four to six months of age can be considered to be a strong sign of early hemiplegia (Golomb et al., 2001). Studies of infants who have sustained an early perinatal stroke from 4 to 7 months corrected age have suggested that until reach to grasp behaviours have emerged, an asymmetry may not be clearly evident so that a hemiparesis may not be confirmed (Duff and Charles, 2004; Duff and Gordon, 2003). Upper limb skills of typically developing infants generally develop in several stages: (1) discovering the hand; (2) visually regarding the hand; (3) visually exploring objects in space; (4) swiping at objects; (5) contacting objects; (6) ineffectively grasping objects; and (7) developing prehensile movements to better grasp objects (White and Held, 1966). These stages of prehension are not consecutive and often overlap (Table 13.1). Prior to the onset of reach, infants have been observed to demonstrate prehensile movements that provide multimodal input about their upper limb function within their environment and sensorimotor experiences that provide early motor programmes for upper limb control (Eyre et al., 2001; Thelen and Smith, 1994; von Hofsten and Ronnqvist, 1993). Grasping involves the shaping and co-ordinated movements of fingers and rotation of the wrist in a manner that anticipates the size, shape and physical features of the target object (Jeannerod, 1997). Table 13.1 Presumed timing of development of reaching, grasping and releasing in infants Sources: Gallahue and Ozmun (2002); Thelen et al. (1993); and von Hofsten et al. (1998). All the components of prehension, including visual regard, reach, grasp, manipulation, pulling, pushing objects and release, can be impacted by an early brain lesion. In typically developing infants hand preference is strong initially and often varies (e.g. Corbetta and Thelen, 1996; Fagard, 1998). Handedness in infants can be observed when they undertake bimanual tasks (Fagard and Marks, 2000). Switching hand preference while manipulating an object happens early in motor development, prior to 6 months of age (Fagard and Lockman, 2005). There is evidence that fine motor skills such as reaching, grasping and releasing develop at variable and often overlapping time points (see Table 13.1). At 5 months of age, typically developing infants demonstrate preparatory forearm rotation and hand pre-shaping based on a toy’s position, shape and size, which leads to successful grasping (von Hofsten et al., 1998). Infants with early asymmetric brain damage and visual deficits can start to develop maladaptive prehensile skills such as asymmetric reaching, increased forearm pronation, ineffective opening and pre-shaping of the hand to the toy. These maladaptive prehensile skills result in inefficient manipulation (such as contacting and grasping toys) and difficulty releasing objects. There is some evidence to suggest that for infants with early brain lesions, important phases of sensorimotor reorganization occur during their first year of life (Eyre et al., 2007). After a brain lesion has occurred, development of the damaged cortex is compromised and its remaining contralateral corticospinal (CS) pathways that connect the damaged cortex to the impaired upper limb stop developing (Eyre et al., 2007). Eventually, the synaptic space that these pathways initially occupied is taken over by the more active ipsilateral pathways (which connect the intact cortex to the impaired upper limb) (Eyre et al., 2007). Both sets of CS pathways compete for synaptic space, which results in the ipsilateral CS pathway outgrowing the contralateral CS pathway. As a consequence, two main types of brain reorganization can be observed after early asymmetric brain injuries. Ipsilesional reorganization (i.e., reorganization occurring within some spared cortical tissue of the damaged hemisphere) allows for the motor cortex of the damaged hemisphere to become reconnected to the spinal cord, and is usually what is seen in adults following stroke. Contralesional organization (i.e., reorganization occurring in the undamaged cortex) is based on existing ipsilateral motor projections remaining intact, instead of becoming retracted within the first months of life. This specific alternative type of reorganization is possible if the lesion occurs early in development (Staudt et al., 2004). It allows the undamaged cortex to directly control both upper limbs and often involves the dissociation of the primary sensory and motor pathways (Guzzetta et al., 2009; Thickbroom et al., 2001), resulting in limited upper limb functional activity (Staudt et al., 2004). On these grounds, the first three to six months of life following an asymmetric brain lesion appear to be a critical window of opportunity for very early intervention. This intervention could be aimed at maintaining cortical motor control within the impaired hemisphere by activating the damaged sensorimotor (SM) cortex (Eyre et al., 2007), and thus enhancing its competitive ability to develop alongside the intact SM cortex, as well as ameliorating the effects of the lesion on upper limb motor activity (Eyre et al., 2007). A crucial role in predicting the type of functional reorganization is influenced by the degree of involvement of the CS tract. A perilesional reorganization can be unachievable in the case of a massive destruction of the CS tract of one hemisphere. Nevertheless, when some sparing of the tract is present, some other factors are likely to come into play, and early intervention can have the potential to shape cortical reorganization and potentially ameliorate the eventual outcome (Fig. 13.1). Figure 13.1 Diagrammatic representation of ipsilesional versus contralesional reorganization following an asymmetric brain lesion and impact on upper limb function. The extent of primary motor cortex (M1) damage can be the strongest predictor of the type of reorganization in case of very mild (left) or very severe (right) injuries. When M1 damage is of intermediate size (centre), the type of motor reorganization can be harder to predict and is likely to be significantly influenced by intervention. A recent review of studies in a feline model provides support for the initiation of prehensile training in infants before six months of age (Martin et al., 2011). The authors have highlighted the close correlation between the activity of the CS tracts and the strength of the synaptic connections with spinal motor circuits. This supports the hypothesis that early brain damage might initiate a vicious cycle in which damaged CS tracts are competitively disadvantaged for maintaining spinal synapses, resulting in secondary reductions in these connections (Martin et al., 2011). More recently, the same group has tested the ensuing hypothesis that targeted activation of the spared CS tracts should lead to functional improvement, by exploring the effects of early intervention in cats with primary motor cortex (M1) inactivation (Friel et al., 2012). Three experimental groups were studied. In the first group, the limb ipsilateral to inactivation was restrained, forcing use of the contralateral impaired limb, for the month following the inactivation (early restraint alone group with no training of the impaired limb). In the second group, the early restraint was supplemented with daily training of a reaching task with the contralateral forelimb (early restraint+training). In the third group, both restraint and training were postponed to feline adolescence (late restraint+training). Outcome was measured at three levels by analysing: (1) CS tract spinal connections; (2) M1 motor maps; and (3) motor performance. Interestingly, restraint alone was able to restore CS tract connectivity yet failed to impact on M1 motor maps or motor function, while late training impacted on both CS tract connectivity and motor maps (however, it failed to induce significant functional recovery). The only intervention resulting in all three measures of outcome was the one based on early restraint combined with training. Altogether these findings suggest that in order to achieve significant motor improvement, a complex network of integrated functions of the CS system needs to be re-established, which targets intervention at multiple hierarchical levels. The importance of a multilevel network in the reorganization of the CS system has been suggested by recent work in humans with congenital hemiplegia. Evidence from advanced diffusion imaging has suggested that the developing connectivity and symmetry of the thalamocortical pathways connecting M1 with the motor thalamus is at least as important as the symmetry of the CS tracts for upper limb unimanual capacity and bimanual co-ordination in children with congenital hemiplegia (Rose et al., 2011). Our group studied 16 children with congenital hemiplegia, of whom nine were classified as having periventricular leukomalakia and seven were classified as having predominantly deep grey matter lesions, according to the Krägeloh-Mann qualitative scheme (Krägeloh-Mann and Horber, 2007). Advanced diffusion imaging utilizing the HARDI model (high angular diffusion imaging) was performed to elucidate the symmetry in the CS (motor) and the thalamocortical (sensorimotor) tracts (Fig. 13.2, Table 13.2). Surprisingly, the sensorimotor thalamic tracts were more significantly correlated with paretic hand functions than were the CS tracts. These data suggest that functional outcome is not only related to the integrity of the CS tract (the final output) but rather to the integrity of a wider neural integrated network. Our data also support the concept that the motor system requires feedback from sensory systems to shape the development of the motor cortex and efferent motor pathways (Eyre et al., 2007; Rose et al., 2011). To date, upper limb rehabilitation has focused primarily on interventions to promote activation of the motor tracts with little regard to the preservation and balance of input to the sensory tracts. Table 13.2 Corticothalamic tract (CTT) pathways were more highly correlated with baseline hand function than corticospinal tract (CST) pathways Figure 13.2 Advanced diffusion imaging utilizing the HARDI model. Top left is motor cortex (pre-+post-central) to brainstem through the posterior limb of the internal capsule (PLIC), top right is motor cortex (pre-+post-central) to brainstem through the thalamus. Bottom row shows cross-sectional area of the same tracts as above at the level of PLIC/thalamus. Upper limb rehabilitation for school-aged children with hemiplegia focuses on improving unimanual and bimanual function and enhancing participation (World Health Organisation, 2001). Our recent meta-analysis of all non-surgical interventions (Sakzewski et al., 2009) provided evidence for modest improvements in unimanual and bimanual co-ordination using various models of constraint-induced movement therapy (CIMT) (Hoare et al., 2010), bimanual intensive training (Charles and Gordon, 2006), a combination of CIMT and bimanual training (Aarts et al., 2011), and adjunctive use of intramuscular botulinum toxin A (BoNT-A) injections combined with upper limb training (Hoare et al., 2010). While early interventions for infants at risk of developing congenital hemiplegia are considered to be very important, the reality is that rehabilitation programmes commonly do not commence until six months of age (Eyre et al., 2007). This may be due to a delayed diagnosis, for those infants not showing early acute signs of brain damage, or to a lack of consensus on the safety and efficacy of early interventions, both of which prevent early intervention from being included in service programmes. To date, no review has investigated the efficacy, feasibility, compliance and impact of upper limb interventions in improving upper limb motor development specifically for infants and young children aged less than 3 years with early brain injury. A recent systematic review of non-surgical interventions (including modified CIMT, bimanual training, goal-directed occupational therapy (OT) and occupational performance-based OT) has examined the efficacy for improvements in unimanual capacity of the impaired limb, bimanual co-ordination and the amount and quality of hand use in randomized clinical trials for infants and toddlers up to 2.5 years of age with asymmetric brain lesions (Perez et al., unpublished data). In three systematic reviews and 17 randomized clinical trials only six per cent of the 1446 participants with unilateral CP were aged less than 2.5 years. In this systematic review nine randomized controlled trials (RCTs) used modified constraint-induced movement therapy (mCIMT, modified for a paediatric population) in samples including infants up to 2.5 years of age (Aarts et al., 2010; Al-Oraibi and Eliasson, 2011; DeLuca et al., 2006; Eliasson et al., 2011; Facchin et al., 2011; Smania et al., 2009; Taub et al., 2011; Wallen et al., 2011; Xu et al., 2011, ) with the total dose varying from 16 hours to 210 hours. Four studies of mCIMT (Aarts et al., 2010; Al-Oraibi et al., 2011; Eliasson et al., 2011; Wallen et al., 2011) found a small treatment effect on bimanual co-ordination for CIMT compared with control groups (OT, physiotherapy [PT], neurodevelopmental therapy [NDT]). Only one study found that mCIMT effects were sustained for the treatment group at 17 weeks follow-up in an ecologically delivered programme of constraint with activity-based practice (Eliasson et al., 2011). One study of CIMT found a clinically important change post-treatment for amount of use and quality of movement using constraint of the unimpaired limb with a cast and shaping to train the impaired limb (DeLuca et al., 2006). Four RCTs used intramuscular botulinum toxin A (BoNT-A) injections for forearm muscles with spasticity interfering with function as an adjunct to goal-directed training (Kanellopoulos et al., 2009; Lowe et al., 2007; Olesch et al., 2010; Wallen et al., 2007). As yet, safety and efficacy of intramuscular BoNT-A is not determined for use in infants with congenital hemiplegia less than 2 years of age. The potential for adverse events (although short acting and reversible), as well as muscle weakness and atrophy in school-aged children (Barber et al., 2011; Gough et al., 2005), promotes caution for the use of neuromuscular blockage of the overactive muscles in young infants with asymmetric brain lesions under two years of age. In our systematic review, none of the study populations solely comprised participants under 2.5 years of age (Perez et al., unpublished data). Seven of the 17 RCTs reported adverse events, some of which were thought to be associated with the intervention, including tolerating CIMT (Wallen et al., 2011), physical symptoms that may have been associated with BoNT-A injections or the conscious sedation (Wallen et al., 2007). As it was not possible to separate the number of adverse events related to participants under the age of 3 years, the feasibility and compliance of these interventions for this younger age group is unclear. The existing evidence suggests small effects of CIMT with activity-based practice and shaping to improve unimanual capacity and bimanual co-ordination (Perez et al., unpublished data).
Very early upper limb interventions for infants with asymmetric brain lesions
The problem
Definitive diagnosis of hemiplegia
Critical periods of typical upper limb motor development
Cortical reorganization after an early brain lesion: a critical window
Current evidence for early upper limb interventions for infants with hemiplegia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






