Chapter 22 Posterolateral Ligament Injuries
Diagnosis, Operative Techniques, and Clinical Outcomes
INDICATIONS
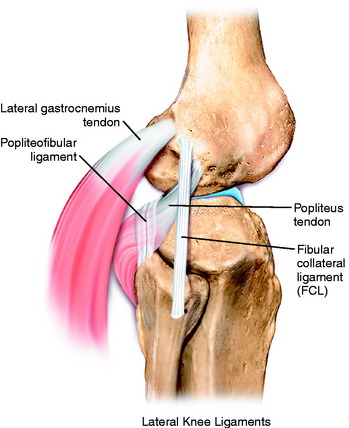
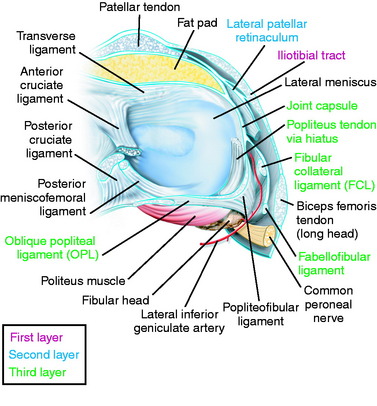
The primary soft tissue stabilizing structures of the lateral and posterolateral (PL) aspect of the knee joint are the fibular collateral ligament (FCL) and popliteus muscle-tendon-ligament unit (PMTL), including the popliteofibular ligament (PFL) and posterolateral capsule (PLC) shown in Figure 22-1. These structures function together to resist lateral joint opening (LJO), posterior subluxation of the lateral tibial plateau with tibial rotation, knee hyperextension, and varus recurvatum.11,12,31,32,47,54
The mechanism of injury may be contact or noncontact and usually involves a combined varus and hyperextension joint displacement. The proper management of injuries involving the PL structures requires knowledge of the complex anatomy and potential variations that may exist, the function of the major soft tissue stabilizers, appropriate diagnostic techniques, and surgical options for reconstruction. Isolated PL injuries are rare; however, on occasion, an avulsion fracture at the femoral attachment occurs requiring internal fixation.24 PL injuries are frequently accompanied by anterior cruciate ligament (ACL) or posterior cruciate ligament (PCL) ruptures.1,8,23
Although the incidence of PL injury is unknown (owing to misdiagnosis or failure to detect the injury), the consequences of untreated PL ruptures are readily apparent. Chronic deficiency of the PL structures may be a factor in the failure of cruciate reconstructions34,36,42 and may also play a role in the development of gait abnormalities and giving-way.43,45,51 The detection and proper treatment of these problems is critical, because failure to properly treat all of the abnormalities may result in a poor outcome. The patient will complain of a varus type of instability with LJO during stance phase and show either a neutral or a valgus alignment. The abnormal LJO during stance phase is always greater than that detected on the varus stress test. The patient may demonstrate the abnormal LJO by producing a varus loading at the knee joint while standing.
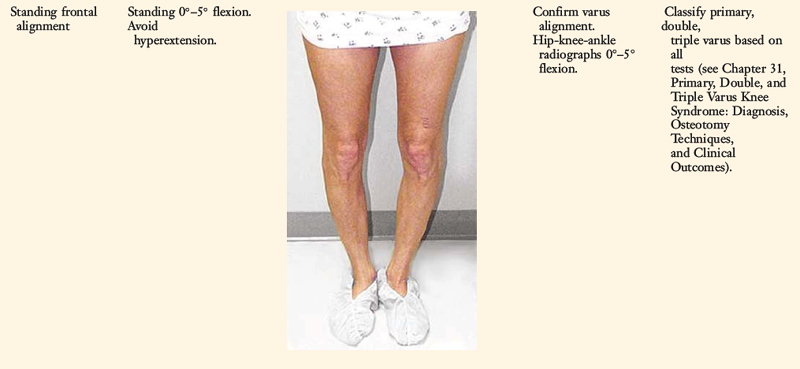
Knees that fulfill the double or triple varus diagnosis criteria (varus osseous malalignment with increased LJO, external tibial rotation, varus recurvatum, and knee hyperextension [see Chapter 31, Primary, Double, and Triple Varus Knee Syndrome: Diagnosis, Osteotomy Techniques, and Clinical Outcomes])40 require high tibial osteotomy (HTO) first, followed approximately 6 months later with an appropriate PL reconstruction. In many instances, an ACL or PCL deficiency also exists, which is corrected at the time of the PL reconstruction.
CONTRAINDICATIONS
Contraindications to PL reconstruction are findings of less than 5 mm of increased lateral tibiofemoral joint opening and less than 10° of increased external tibial rotation. These findings are frequently noted in knees with associated varus osseous malalignment (double varus knees) that are candidates for HTO (see Chapter 31, Primary, Double, and Triple Varus Knee Syndromes: Diagnosis, Osteotomy Techniques, and Clinical Outcomes).33 Correction of the varus malalignment promotes physiologic remodeling and shortening of the PL structures, decreasing the abnormal LJO and external tibial rotation and thus negating the need for a PL operative procedure.
Critical Points CONTRAINDICATIONS
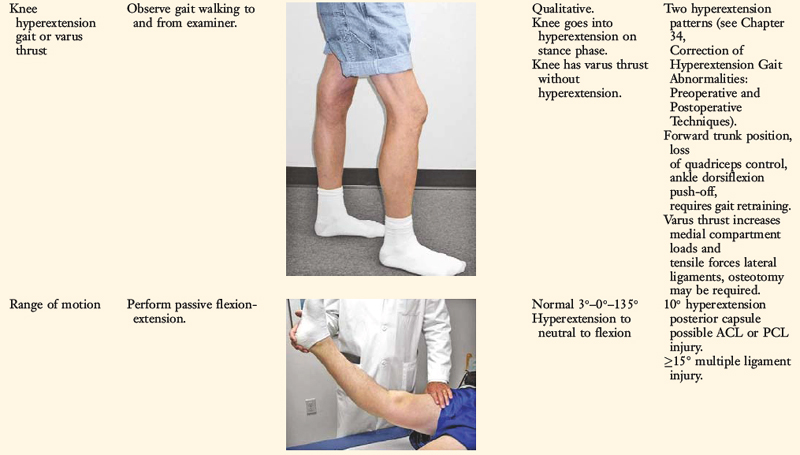
Patients with varus malalignment who do not undergo HTO and have associated chronic insufficiency of the PL structures are not candidates for a PL procedure. Untreated varus osseous malalignment is a cause of failure of PL reconstructions.39 In many cases, a knee hyperextension gait abnormality also exists that must be corrected before surgery with a specific gait-retraining program described in Chapter 34, Correction of Hyperextension Gait Abnormalities: Preoperative and Postoperative Techniques.43 Failure to correct a hyperextension gait abnormality places PL reconstructions at risk for failure owing to the excessively high tensile forces placed on the PL soft tissues upon weight-bearing after surgery. Gait retraining usually decreases abnormally high knee extension and adduction moments to normal values.43
CLINICAL EVALUATION
Critical Points CLINICAL EVALUATION
History
Tibiofemoral Rotation Dial Test
One frequent patient presentation is a failed ACL or PCL reconstruction owing to untreated PL insufficiency. Another patient presentation is a chronic varus osseous malalignment and underlying ACL insufficiency in which, over time, interstitial stretching and slackening of the PL structures occurred.33,40 In these cases, HTO unloads the PL soft tissues to the extent at which physiologic remodeling and shortening occur and PL reconstruction is not required.40
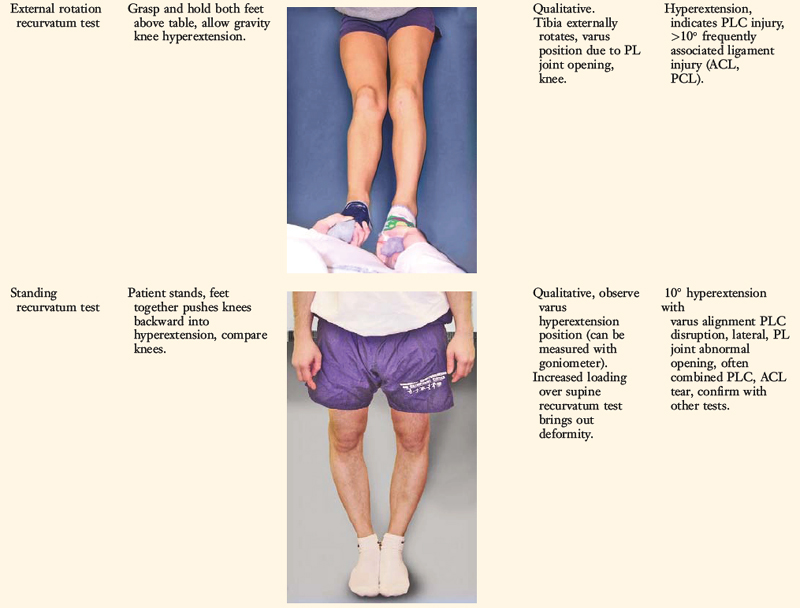
A comprehensive physical examination is required, including assessment of knee flexion and extension, patellofemoral indices, tibiofemoral crepitus, tibiofemoral joint line pain, and gait abnormalities. Pain in the medial tibiofemoral compartment occurs owing to increased compressive forces related to varus osseous malalignment. Pain in the PL soft tissues may occur from increased soft tissue tensile forces due to a varus thrusting gait pattern. The abnormal knee hyperextension involves increased extension in the sagittal plane and is often accompanied by a varus alignment in the coronal plane, which has been described as a varus recurvatum alignment. Together with a varus osseous malalignment, this is referred to as a triple varus knee (see Chapter 31, Primary, Double, and Triple Varus Knee Syndromes: Diagnosis, Osteotomy Techniques, and Clinical Outcomes). Patients with chronic PL insufficiency have varying amounts of altered gait mechanics and knee hyperextension. Some individuals may present with a markedly abnormal gait that is severely disabling and limits ambulation. Other patients may have a less noticeable alteration because the abnormal knee hyperextension occurs only after prolonged walking and muscle fatigue. The abnormal gait pattern is characterized by excessive knee hyperextension during the stance phase, which does respond to gait retraining that initiates normal stance phase flexion (see Chapter 34, Correction of Hyperextension Gait Abnormalities: Preoperative and Postoperative Techniques). Subjective complaints of giving-way during routine daily activities, along with severe quadriceps atrophy, often accompany this gait abnormality.
The surgeon must determine all of the abnormal translations and rotations in the knee joint. The ligament injuries that result in knee hyperextension and varus recurvatum frequently involve not only the PL structures but also other ligament and capsular structures. The biomechanical and kinematic studies that form the basis for the interpretation and diagnosis of the manual stress tests are described in Chapter 20, Function of the Posterior Cruciate Ligament and Posterolateral Ligament Structures.
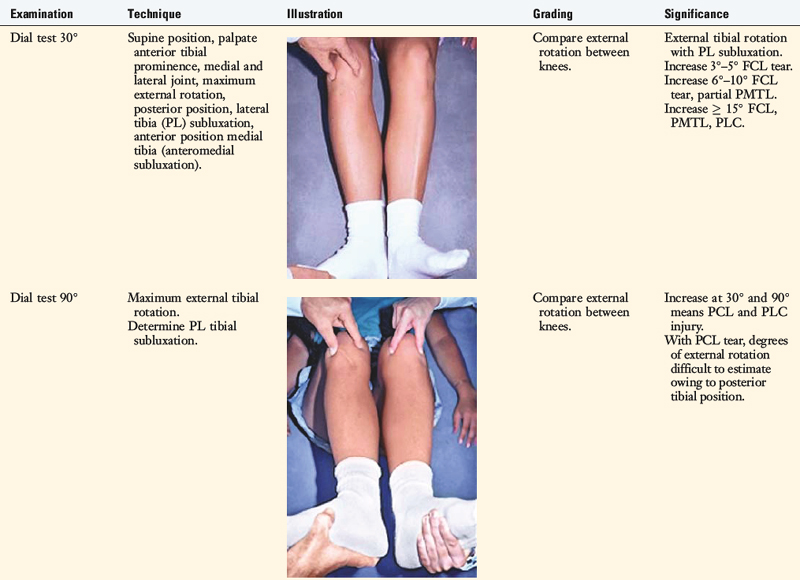
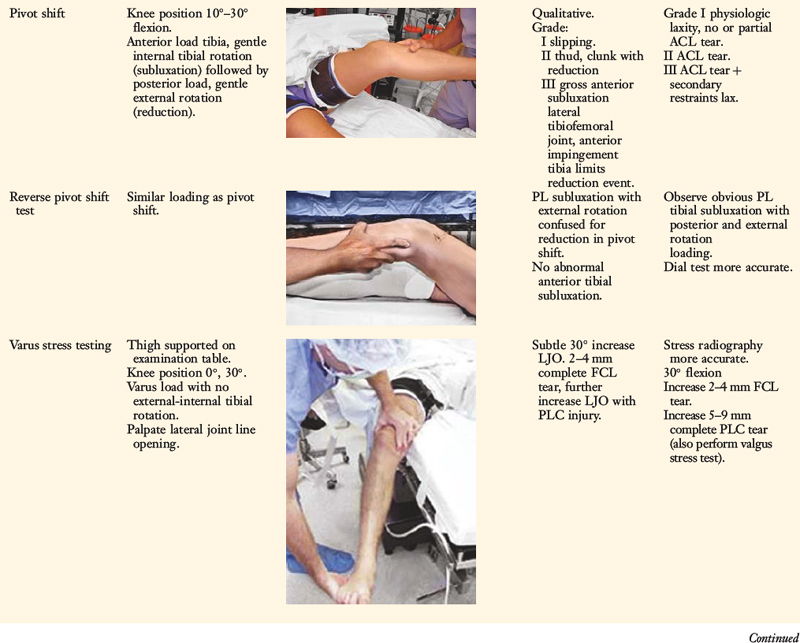
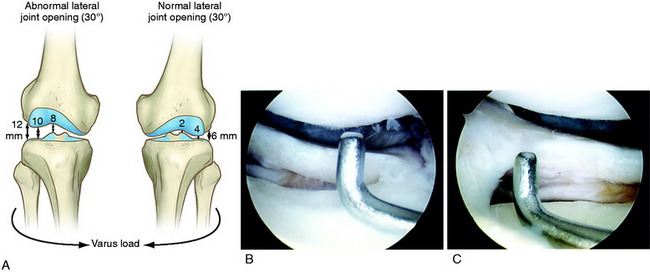
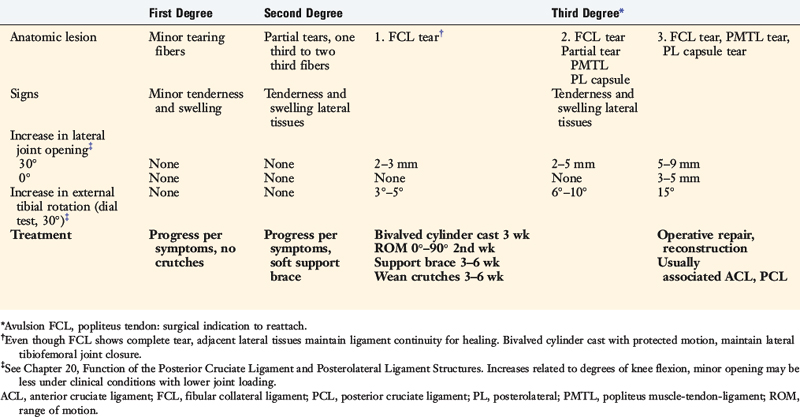
The increases in LJO and external tibial rotation shown in Table 22-1 are only approximations of what would be expected with clinical injury to the PL structures. Importantly, an increase of only a few millimeters (2–5 mm) in LJO occurs with complete rupture of the FCL, whereas an increase of 5 to 9 mm occurs with complete rupture of all the PL structures (FCL, PMTL, PFL). These values are based on biomechanical studies discussed in Chapter 20, Function of the Posterior Cruciate Ligament and Posterolateral Ligament Structures, under moderate varus loads (20 Nm). LaPrade and colleagues20 conducted a cadaveric study in which lateral stress radiography was applied at 12 Nm (on an experimental apparatus) and the increase in LJO over the intact state was compared with that measured during a clinician-applied load after an isolated FCL rupture and a combined FCL, PMTL, PFL rupture. Compared with the intact state, LJO induced by the clinician-applied load increased by 2.7 mm (isolated FCL rupture) and 4.0 mm (combined PL rupture). However, the mean values showed a wide standard deviation and variation between specimens, making extrapolation to the clinical setting difficult. In addition, the lateral joint space measurement showed wide confidence intervals. For an isolated FCL rupture, the mean lateral gap distance was 10.99 mm (confidence interval [CI], 7.8–14.3 mm) and for the combined PL rupture, the mean distance was 12.2 mm (CI, 9.3–15.2 mm). This amount of overlap indicates that it would not be possible to accurately separate an FCL rupture alone from a combined PL injury. The measurements are important and useful in providing the clinician with a baseline in interpreting lateral stress radiographs. The gap test measurement at arthroscopy described extensively in this book is based also on these types of approximations. The gap test is based on the joint separation between articular cartilage seen at arthroscopy, and not the cortical separation on a stress radiograph. Even so, the measurements are somewhat equivalent as to the increase in the amount of millimeters with PL injuries. For example, Figure 22-2 shows an approximately normal lateral gap of 4 mm at the closest point of the lateral compartment at arthroscopy. An increase of only 6 mm results in 10 mm of absolute opening at the closest point, or 12 mm at the periphery, which is viewed as a positive gap test and indicative of injury to the PL structures. Fortunately, in most knees, these are the lesser values and it is more common that the lateral gap exceeds these measurements, indicating that concurrent PL reconstruction is necessary.
An increase in external tibial rotation may occur with anterior subluxation of the medial tibial plateau, posterior subluxation of the lateral tibial plateau, or a combination of both subluxations. The dial or spin rotation test, which the senior author developed, allows a diagnosis of tibial rotatory subluxations of the medial and lateral tibiofemoral compartments at 30° and 90° of knee flexion (see Table 22-1). Other variations of this test have been described.6,44,55
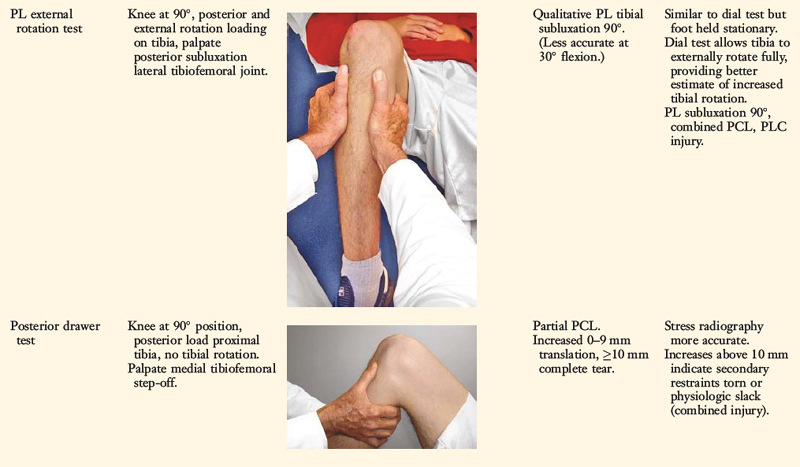
The position of the medial and lateral tibial plateau is assessed at the starting position (neutral tibial rotation) with the knee flexed to 30° and 90° and at the final position with the tibia in maximal external rotation. The examiner palpates the position of the medial and lateral tibial plateau, which is compared with the normal knee to assess whether a subluxation (anterior or posterior) of the medial or lateral tibial plateau is present. An increase in internal tibial rotation occurs with medial ligament and PCL disruption (see Chapter 20, Function of the Posterior Cruciate Ligament and Posterolateral Ligament Structures). The axis of tibial rotation is observed in the involved knee and compared with the normal knee to detect a shift in the medial or lateral tibiofemoral compartment during tibial rotation. It is not recommended that this test be performed in the prone position because the tibiofemoral joint cannot be accurately palpated to distinguish an anteromedial from a PL tibial subluxation.
The use of the dial test in knees with PCL ruptures requires maintenance of a normal anatomic tibiofemoral position. This is accomplished by applying a gentle anterior translation, loading the ACL in both limbs, during the external tibial rotation. It is still necessary to use the supine position so that the examiner can palpate the tibiofemoral position.52 The dial test is less accurate with a PCL rupture, because it is difficult to compare limbs, and other tests to be described (LJO, gap test at arthroscopy, varus recurvatum) for the integrity of the PL structures need to be carefully assessed.
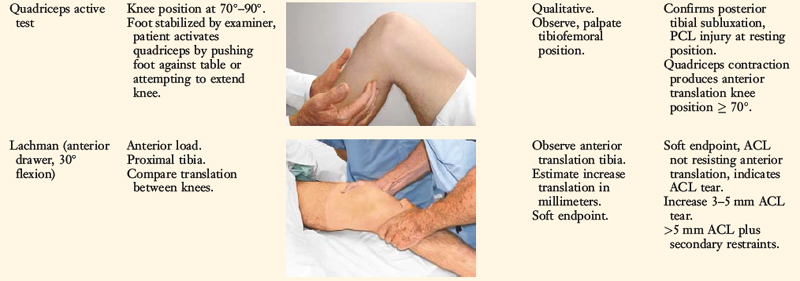
Posterior stress radiographs are obtained in patients with PCL ruptures, especially those in which the distinction of a partial versus a complete PCL deficiency is difficult to determine on clinical examination.14 A lateral PCL stress radiograph is taken of each knee at 90° of flexion. The limb is placed in neutral rotation with the tibia unconstrained and the quadriceps relaxed, and 89 N force applied to the proximal tibia. Measurement is made of the millimeters of posterior tibial translation in both knees. Knees with 10 mm or more of increased posterior tibial translation are considered candidates for PCL reconstruction.
Full standing radiographs of both lower extremities, from the femoral heads to the ankle joints, are done in knees with varus lower extremity alignment. The mechanical axis and weight-bearing line are measured to determine whether HTO is indicated.10
Patients complete questionnaires and are interviewed for the assessment of symptoms, functional limitations, sports and occupational activity levels, and their perception of the overall knee condition according to the Cincinnati Knee Rating System (CKRS; see Chapter 44, The Cincinnati Knee Rating System).2
CLASSIFICATION AND TREATMENT OF PARTIAL TO COMPLETE PL INJURIES
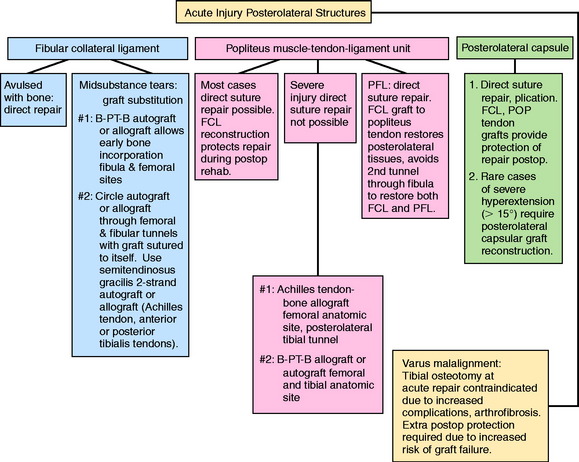
The classification and treatment of first-, second-, and third-degree acute PL injuries is detailed in Table 22-2. It is important to diagnose partial tears of the PL structures, with a mild to moderate increase in LJO and external tibial rotation, to allow protection and maintain lateral tibiofemoral joint closure in the initial 3 weeks to allow “stick-down” and healing of lateral soft tissues. This program is similar to that recommended for medial ligament ruptures (see Chapter 24, Medial and Posteromedial Ligament Injuries: Diagnosis, Operative Techniques, and Clinical Outcomes).
PREOPERATIVE PLANNING: TIMING OF SURGERY
Acute Injuries
There is a distinct advantage for repairing completely disrupted PL structures and meniscal attachments in acute injuries (Fig. 22-3). At the time of surgery, extensive disruption of these structures is observed. Careful dissection is required to identify anatomic tissue planes and maintain an intact vascular and neural supply. The so-called golden period to perform an acute surgical repair is within 7 to 14 days of the injury. After this time, scar tissue will obliterate tissue planes and make the dissection and repair difficult.
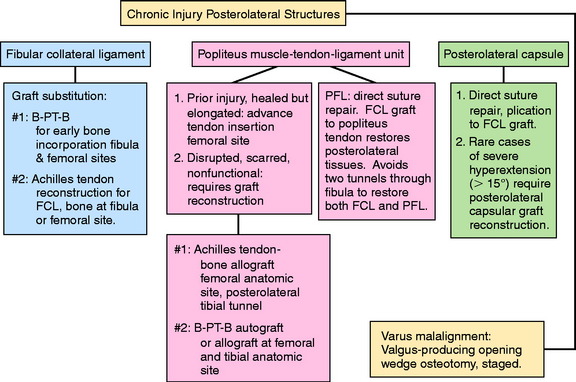
Chronic Injuries
Patients with chronic knee injuries that present with severe muscle atrophy require several months of preoperative rehabilitation. Patients with a hyperextension gait abnormality must complete a gait-retraining program,43 described in detail in Chapter 34, Correction of Hyperextension Gait Abnormalities: Preoperative and Postoperative Techniques. This program is done in addition to lower extremity muscle strengthening exercises. In the authors’ experience, patients will convert to a more normal gait pattern after 4 to 6 weeks of training. More time is required for severe quadriceps atrophy before surgical intervention.
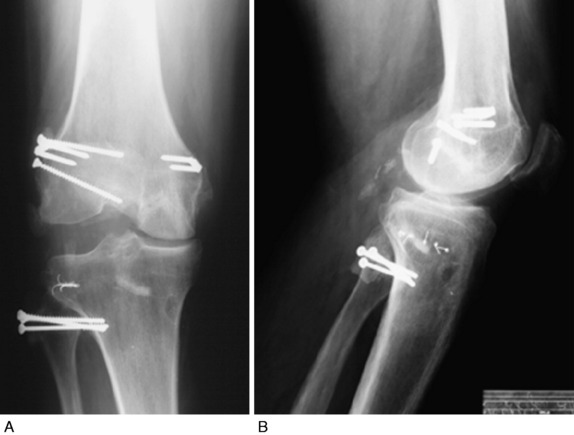
Varus osseous malalignment must be corrected before chronic PL reconstruction, as described previously. Failure to address this malalignment will greatly increase the risk of failure of any PL procedure (Fig. 22-4). In some cases in which the PL deficiency is due to interstitial stretching of the tissues and not a traumatic rupture, a simplified proximal advancement of the PL structures may be performed with the HTO. In anatomic PL reconstructions, the ligament surgery is staged after healing of the HTO. The indications for the various PL procedures are described in detail under the “Operative Treatment of Acute PL Ruptures” and “Operative Treatment of Chronic PL Ruptures” sections.
Patients who have undergone prior lateral meniscectomy and who demonstrate early tibiofemoral arthritis are considered for lateral meniscus transplantation.41
Cruciate Graft Reconstruction
The majority of patients who undergo PL reconstruction require a concomitant ACL or PCL reconstruction (Fig. 22-5). The appropriate grafts for the cruciate procedures should be determined; autogenous tissues with bony fixation are preferred. However, the surgeon should ensure that B-PT-B and Achilles tendon–bone (AT-B) allografts are available the day of surgery. These will be required if autogenous tissue is unavailable or not suitable for the PL or cruciate procedures.
INTRAOPERATIVE EVALUATION
All knee ligament tests are performed after the induction of anesthesia in both the injured and the contralateral limbs. The amount of increased anterior tibial translation, posterior tibial translation, LJO, and external tibial rotation is documented. A thorough arthroscopic examination is conducted, documenting articular cartilage surface abnormalities (see Chapter 47, Articular Cartilage Rating Systems) and the condition of the menisci.46
Critical Points INTRAOPERATIVE EVALUATION
The gap test is done during the arthroscopic examination.40 The knee is flexed to 30° and a varus load applied. A calibrated nerve hook is used to measure the amount of lateral tibiofemoral compartment opening (see Fig. 22-2). Knees that have 12 mm or more of joint opening at the periphery of the lateral tibiofemoral compartment require a PL reconstructive procedure.
OPERATIVE TREATMENT OF ACUTE PL RUPTURES
Operative Setup and Patient Positioning
Identification of Ligament and Soft Tissue Rupture Pattern
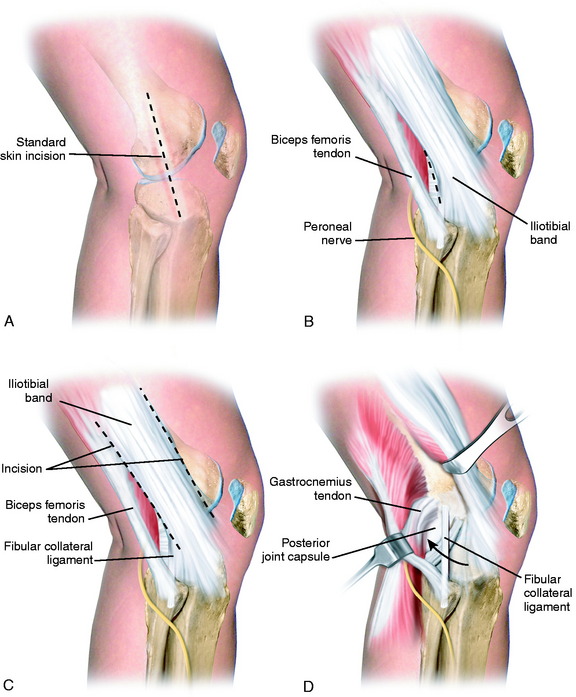
A 10- to 12-cm skin incision is made in a straight line centered over the joint line and 1 cm posterior to the iliotibial band (ITB) attachment at the tibia (Fig. 22-6A). After careful mobilization of the skin flaps, the ITB, biceps tendon, and lateral structures are encountered.
In the majority of knees, the ITB will be intact or demonstrate only partial tearing. In select cases, the ITB will be completely disrupted at the joint line or avulsed off its tibial attachment at Gerdy’s tubercle. Posteriorly, there are capsular attachments of the ITB, including fascial attachments to the short head of the biceps femoris, which are identified for later repair if torn. If the ITB is intact, an incision is made along its posterior border to allow visualization of all of the underlying structures (see Fig. 22-6B).
The lateral capsular tissues and meniscal attachments are the next structures visualized. A vertical incision is made into the anterior third of the capsule and extended to the lateral meniscus just anterior to the popliteus tendon attachment. The popliteus tendon and meniscus attachments at the femoral popliteal recess are identified. Frequently, it is necessary to repair the superior and inferior meniscal fasciculi (Fig. 22-7) to restore meniscal attachments to the lateral meniscus. Careful varus stress is placed on the knee joint to allow inspection of the lateral meniscus attachments and tibiofemoral articular cartilage. On occasion, an additional anterior incision is required for visualization of underlying anatomy (see Fig. 22-6C).
The fibular head and attachments of the biceps femoris short and long head are the next structures visualized, which have been described in detail in Chapter 2, Lateral, Posterior, and Cruciate Knee Anatomy. The two tendinous components (direct and anterior arms) and one of the fascial components (lateral aponeurotic expansion) make up the key portion of the long head anatomy. The other fascial components are the reflected arm and the anterior aponeurotic expansion.
The most proximal component is the reflected arm. It originates just proximal to the fibular head and ascends anteriorly to insert on the posterior edge of the ITB. The direct arm inserts onto the PL edge of the fibula just distal to the tip of the styloid. A portion of the anterior arm inserts onto the lateral aspect of the fibular head, and the rest continues distally just lateral to the FCL. Portions of the anterior arm ascend anteriorly forming the lateral aponeurotic expansion that attach to the posterior and lateral aspects of the FCL. Here, a small bursa separates the anterior arm from the distal fourth of the FCL. The anterior arm thus forms the lateral wall of this bursa (see Fig. 2-12). This is an important surgical landmark, because a small horizontal incision can be made here, 1 cm proximal to the fibular head, to enter this bursa and locate the insertion of the FCL into the fibular head. The anterior arm then continues distally over the FCL, forming the anterior aponeurosis, which covers the anterior compartment of the leg. The primary areas of injury are tendon avulsions off of the fibula, which often have a major osseous component that can be repaired. In addition, the fascial extensions anteriorly and laterally are repaired.
The short head of the biceps courses just deep (or medial) and anterior to the long head tendon, sending a majority of its proximal muscular fibers to the long head tendon itself.53 It has six distal attachments, described in detail in Chapter 2, Lateral, Posterior, and Cruciate Knee Anatomy. The most important attachments are that of the direct arm, the anterior arm, and the capsular arm.
The interval between the posterior capsule and the gastrocnemius tendon is entered just above the fibula, similar to the exposure for a lateral meniscus repair. This exposes the popliteus muscle tibial attachments, popliteus muscle-tendon junction, PFL, popliteus tendon attachment at the femur, and fabellofibular ligament (extension of short head biceps attachments) (see Fig. 22-6D).
In dissection studies, LaPrade and coworkers19 described a fabellum (osseous or cartilagenous) present in all specimens. This structure forms an attachment for the oblique popliteal ligament and fabellofibular ligament, which along with the posterior capsule, are important restraints for limiting knee hyperextension. Although individual capsular components and structures are difficult to discern with extensive capsular ruptures, it is important to repair disrupted posterior capsular tissues after completion of the initial dissection.
Peroneal Nerve Identification
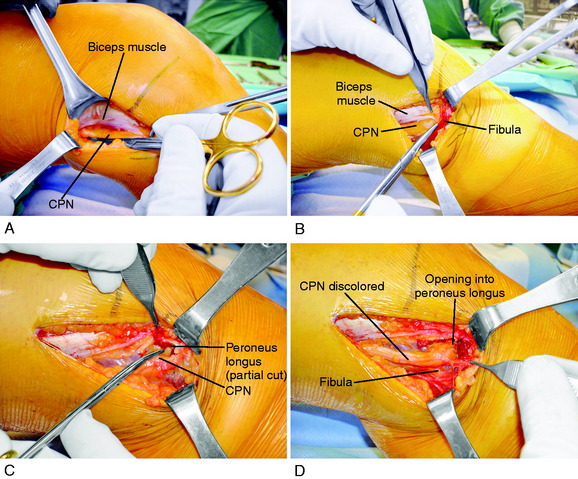
It is important at the initial stages of the dissection to palpate and determine the location of the CPN. To expose the CPN, it is safest to begin in the proximal aspect of the operative exposure. A large retractor is used to elevate the muscular portion of the biceps femoris, placing the fascial tissues beneath the biceps muscle under gentle tension. This gentle upward displacement of the biceps muscle is key to visualize and dissect the CPN, because its normal curviform undulations are removed and it assumes a straighter appearance (Fig. 22-8A). The investing crural fascia is incised over the CPN to the fibula.
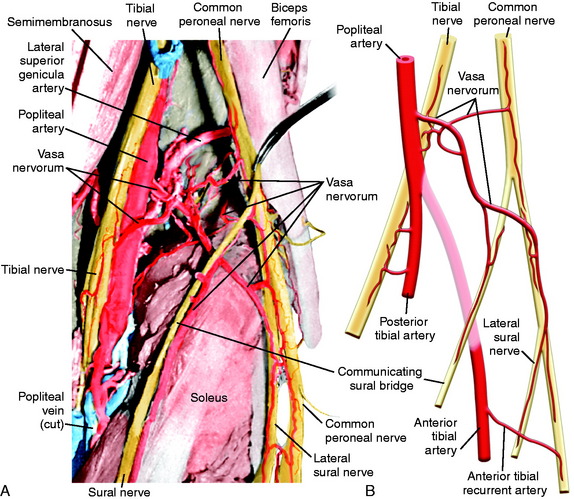
The CPN and its branches are not removed from their normal anatomic position to avoid damaging the delicate blood supply, particularly in the region where the CPN approaches and then passes around the fibular neck. Kadiyala and associates16 reported measurements in cadaveric specimens of the blood supply to the CPN in the popliteal fossa and fibular neck region. These authors hypothesized that the susceptibility of the CPN to injury or lack of a response to operative treatment when injured may be related to deficiencies in intraneural and extraneural vascular supply and anastomoses.
The most common source of blood supply to the proximal portion of the CPN is a direct branch of the popliteal artery. This branch divides into proximal and distal anastomotic vessels that run in the connective tissue sheath of the nerve and anastomoses with the anterior recurrent tibial artery. The vessels, located in the epineurium, give rise to many small vessels of fine caliber, which extend 20 to 30 mm within the substance of CPN. It is important not to disturb this blood supply. Kadiyala and associates16 noted that the blood supply of the CPN was somewhat sparse with poor vascularization. A connection of the vasa nervorum was not found from the geniculate arteries, but occasional contributions from muscular branches were recognized (Fig. 22-9).
Bottomley and colleagues3 reviewed the anatomic position of the CPN in 54 patients who had damage to the PL structures. The CPN was noted to be displaced out of its normal position in 16 of 18 patients who had biceps avulsions or associated fibular head fractures. These authors advised that the surgeon should expect an abnormal nerve position on surgical exploration in knees with bone or soft tissue avulsion from the fibular head and the potential for iatrogenic damage.
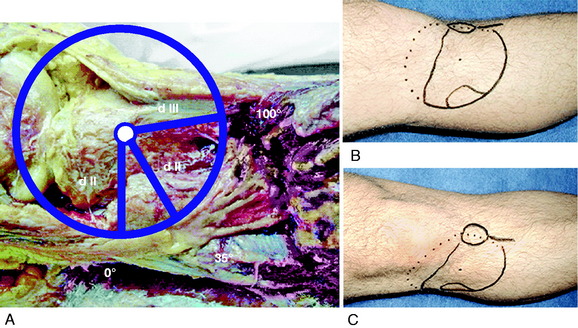
Rubel and coworkers49 conducted an anatomic investigation of the CPN in 31 cadaveric limbs by dissecting the CPN to its intramuscular branches. The authors described Gerdy’s safe zone as the area where the CPN and anterior recurrent branch defined an arc with an average radius of 45 mm. The distance between the fibular head and Gerdy’s tubercle was used to determine the radius of the safe zone. Therefore, this region in the proximal aspect of the tibia is advantageous for surgical exploration, because damage to the peroneal nerve and its branches is avoided (Fig. 22-10). The CPN divides into three branches as it enters the anterolateral musculature, with the anterior recurrent branch more proximal to the superficial and deep peroneal branches.
Dellon and associates9 reported on the anatomic variations of the CPN at the fibular head in 29 cadavers (bilaterally) and 65 patients treated with a CPN decompression for symptoms. Three possible anatomic variants were described that require attention and decompression in chronic neuropathies for a successful outcome. First, the superficial fascia of the superficial head of the peroneus longus muscle is divided by a proximal and distal transection of the fascia (found in 30% of cadavers and 78% of patients; see Fig. 22-8B). Second, when the peroneus longus muscle at the fibular neck is partially incised adjacent and superior to the CPN and the peroneus muscle lifted anteriorly, a fibrous band may be found that requires release (soft tissue restriction found in 43% of cadavers and 20% of patients; see Fig. 22-8C). Third, there may be a fibrous connection between the peroneus longus and the soleus muscle requiring division (found in 9% of cadavers and 6% of patients). These authors advise that after CPN decompression, the surgeon’s index finger should be able to gently pass along the CPN and into the anterolateral compartment (see Fig. 22-8D).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree