14 Intramuscular stimulation (IMS)
Introduction
Gunn Intramuscular Stimulation (Gunn-IMS) is a technique for the treatment of myofascial pain syndrome based on a comprehensive diagnostic and therapeutic model that identifies the etiology of myofascial pain as neuropathic, i.e. due to disease or dysfunction in the nervous system. Further, it identifies the nerve root as the locus of the pathology, and thus it is a radiculo-neuropathic model. It was developed by C.C. Gunn MD, in the 1970s while treating injured workers and followed from his clinical observations distinguishing those workers who succeeded in returning to work from those who failed to do so (Gunn & Milbrandt 1976a).
Gunn’s model is a derived clinical model. What grew out of the desire to understand his patients’ persistent pain and offer them treatment ended in a completely new way to see and treat this most universal of all human afflictions, pain. He was the first physician ever to recognize the subtle physical examination signs of neuropathy and to describe the pathophysiology of neuropathic pain (Gunn & Milbrandt 1978, Gunn 1980).
Gunn’s work reflects not only the great tradition of empirical science, but also, is built upon the work of great scientists before him. In an example par excellence of the often quoted homage of scientific progress to the work of all who precede one in the history of science and medicine, ‘dwarfs standing on the shoulders of giants’, Gunn realized the pathophysiological explanation for what he observed clinically in the work of Walter Cannon, the distinguished early 20th century physiologist. Cannon’s research on the ‘The Supersensitivity of Denervated Structures, a Law of Denervation’ is an important law of neuropathophysiology that, because of its posthumous publication in the non-scientific literature, had been previously overlooked by the medical community (Cannon & Rosenblueth 1949). While an entire field of basic and animal neuromuscular physiology research has grown out of this work known as ‘Cannon’s Law’ (CL), it had remained a purely academic and non-clinical pursuit limited to the laboratory. Until Gunn, who has built a bridge from the research laboratory to the medical clinic and rescued CL from experimental obscurity to practical posterity.
The prevailing medical-surgical management of persistent spinal and regional musculoskeletal pain is based on what could be called the ‘spondylosis-nociception-inflammation’ model. This model attributes pain to nociceptive and inflammatory etiologies due to altered structure in a normal peripheral nervous system. Typical diagnoses in this model include ‘disc rupture, degeneration and inflammation’, ‘nerve root impingement’, ‘facet joint arthropathy’, ‘rotator cuff tear’, ‘extensor elbow/Achilles tendonitis’, ‘hip bursitis’, ‘patella-femoral dysfunction’, and ‘plantar fasciitis’, to name a few. Diagnosis and treatment decisions in this model are based largely on the structural findings of imaging studies (plain X-ray, CT/MRI, nuclear medicine) or the presumption of inflammation, and myofascial pain syndrome (MPS) is not thought of as bearing any relationship to these entities. This model however, cannot account for many clinically ‘inconvenient’ facts, including the lack of correlation between anatomic findings and pain (Savage et al. 1997; Borenstein et al. 2001) or the absence of exam findings or histological evidence of inflammation (Khan et al. 1999, Alfredson & Lorentzon 2002).
Connecting the dots between radiculopathy, neuropathy and myofascial pain, one could say that Gunn discovered the ‘missing link’ between three entities previously thought of as separate, even disparate. Gunn’s radiculo-neuropathic-myofascial pain (RNMP) model explains many of the failures and paradoxes of the traditional model, and accounts for many of the facts that a nociceptive and inflammatory model alone cannot. These include the common clinical observations of painless nerve impingement, why pain may resolve despite imaging evidence of persistent nerve impingement or electrodiagnostic evidence of ongoing acute denervation, or why pain may persist even after surgical nerve root decompression or in the absence of detectable inflammation. Understanding persistent spinal and regional musculoskeletal syndromes as manifestations of RNMP and not inflammatory in etiology explains the common failure of anti-inflammatory therapy for these conditions. It also explains why strengthening exercise, which normally produces muscle shortening, often fails to relieve pain, and not infrequently worsens it, as it aggravates the already present muscle shortening seen in RNMP syndromes. Alternatively, it explains why such therapies as osteopathic manipulation, myofascial release, stretching, transcutaneous electrical nerve stimulation (TENS), diathermy, therapy, acupuncture, trigger point injection and spinal cord stimulation may be effective. It would predict that muscle relaxant as well as anti-neuropathic medications like gabapentin might be effective (Audette et al. 2005).
Gunn’s identification of myofascial pain as essentially a neuropathic condition leads to additional insights. As a treatment for MPS, Gunn-IMS is hardly different from the other techniques of superficial and deep dry needling described in this textbook, and not only predicts but readily recognizes the efficacy of these approaches. Yet while Gunn-IMS does not differ from dry needling (DN) in much of its technique, or the ‘how’, it does differ substantially from other dry needling techniques in its understanding of the ‘what’, ‘where’, ‘why’ and ‘when’ of MPS. It differs in explaining ‘what’ causes MPS and trigger points and so how to examine the patient and thus ‘what’ for and ‘where’ to look on physical examination. This leads to a rationale for ‘where’ to treat the patient, i.e. in a segmental or radiculoneuropathic pattern of myotomal involvement. In its recognition of MPS as neuropathic, it proposes an explanation of ‘why’ Gunn-IMS, along with many other forms of counter-irritation reflex stimulation are effective in reversing neuropathic supersensitivity. Understanding the time frame for experimental reversal of neuropathic supersensitivity (Lomo & Rosenthal 1972, Lomo & Westgaard 1975), it also provides a ‘when’ – that is, a rationale for the expected length and course of treatment based on the severity of the physical examination findings. These and not the technique per se are what differentiate Gunn-IMS from DN.
Gunn’s model recognizes the ‘myofascial trigger point’ (TrP), but it recognizes the TrP as just one of many clinical manifestations of RNMP. Because it is a radiculopathic model, it predicts the presence of TrPs in a myotomal distribution including the posterior ramus, and recognizes the importance of treating such points. Yet despite these differences Gunn-IMS practitioners share in common with all practitioners who treat MPS the recognition of both the prevalence of MPS and the success of treating it early and properly.
Indeed the most important aspect of Gunn’s contribution is not even necessarily the technique of Gunn-IMS (although important), but that it will hopefully lead to wider recognition by the medical community of the significant incidence and prevalence of MPS in the general population. Epidemiological studies suggest that MPS is an important source of morbidity in the community (Cummings & White 2001), yet it is commonly overlooked in the clinic (Skootsky et al. 1989). This is corroborated by the fact that it is found in 85% of patients seen in chronic pain clinics (Fishbain et al, 1989). By recognizing MPS as a common cause of persistent pain beyond 3 months, the possibility of earlier recognition and proper treatment increases dramatically, and with that, the hope of stemming the epidemic tide of chronic pain that is overwhelming western medical systems. Despite all of the rich resources we have thrown at this problem by pursuing the standard paradigm of ‘spondylosis-nociception-inflammation’: strengthening exercise programs, imaging studies, spinal injections, surgery, multidisciplinary pain clinics, opioids, spinal cord stimulators and pumps, we have ended up with increasing suffering, impairment, opioid dependence, disability and unsustainable costs (Deyo et al. 2009). The only thing we have not done is recognize and treat myofascial pain early and properly. By placing myofascial pain squarely within the pathophysiological schema and thus diagnostic algorithm of spondylotic pain, myofascial pain can be properly seen as the prevalent condition that it is. Clinical presentations of myofascial pain are protean in their manifestations: pain referral, while following general patterns, are individually variable, inconsistent and sometimes enigmatic, and they can be over-shadowed by the non-specific nature of the non-pain complaints referable to autonomic mediation that suggest primary visceral pathology (Fricton et al. 1985). All of these features make it difficult to standardize case definition, thus making diagnosis elusive. Gunn’s model accounts for this variability and provides an objective approach to the evaluation and treatment of these patients. Rather than a possible afterthought when the existing model fails, myofascial pain will hopefully be moved to the forefront of the algorithmic evaluation of pain that persists for more than 3 months.
While Gunn has exploited CL in the service of treating pain primarily, the implications of this law and Gunn’s therapeutic model go beyond the treatment of neuromusculoskeletal pain. While beyond the scope of this chapter, taken to its logical and inevitable conclusion, Gunn’s model proposes a rational basis for the treatment of syndromes caused by the autonomically mediated visceral epiphenomena of segmental radiculoneuropathy, including such varied complaints as vertigo, tinnitus, irritable bowel syndrome, and infertility, to name but a few. Current research interest in the role of the nervous system in chronic, or ‘para-inflammation’, suggests even broader and significant implications of Gunn’s model (Tracey 2002).
Gunn-IMS is a procedure that can carry significant risks, especially when treating deeper paraspinal muscle contractures or anywhere overlying the lungs or near vascular structures. Despite these risks, properly qualified health care providers, both primary care and specialist, can be taught to apply it safely and readily to many of the most commonly encountered clinical problems. In addition, Gunn-IMS, like all DN techniques, is ‘low tech’, inexpensive and easily employed in clinics worldwide. Yet while any practitioner can easily be taught to stick a pin into a muscle, as mentioned previously it is the understanding of ‘what’ may cause the TrP, ‘where’ and ‘how’ to treat the patient, ‘what’ responses are sought by needling, ‘why’ needling is likely effective, and ‘when’, or how often and for how long to treat the patient, that constitute the proper application of Gunn-IMS.
Neurophysiological mechanism of Gunn-IMS
In seeking to understand his clinical findings Gunn found an explanation in Cannon and Rosenblueth’s ‘The Supersensitivity of Denervated Structures, a Law of Denervation’. Following the identification of segmental myalgic hyperalgesia (‘tenderness at motor points’) as a correlate of radiculopathy (Gunn & Milbrandt 1976a), subsequent observations included the heretofore unrecognized neuropathic findings in these patients: increased muscle tone, neurogenic edema, vasomotor disturbances with hypothermia, exaggerated pilomotor and sudomotor reflexes, and dermatomal hair loss (Gunn & Milbrandt 1978).
Cannon & Rosenblueth’s Law is summarized as follows:
Gunn, as a practicing physician, first recognized the clinical manifestations of CL:
In the muscle, the above responses are demonstrated by a lowered threshold to acetylcholine (ACh) inducing a contraction. It has also been shown in both striated and smooth muscle that the surface area of the muscle fiber that is sensitive to ACh increases. That is to say ‘extra-junctional’ areas on the surface away from the zone of innervation, normally the only area receptive to ACh stimulation, now respond to ACh. This phenomenon is detectable 4–5 days after denervation, and reaches a maximum within about a week, at which time the entire surface of the muscle fiber is as sensitive to ACh as the neuromuscular junction (Axelsson & Thesleff 1959).
Another manifestation of denervation supersensitivity in the muscle fiber is the development of spontaneous electrical activity, called fibrillation. In contrast to an action potential in the muscle fiber occurring only in response to the release of neurotransmitter, action potentials now occur spontaneously due to changes in membrane potentials and conductivity. In electromyography, spontaneous depolarizations are called ‘denervation potentials’, and reflect loss of motor innervation; they are seen in diseases of the anterior horn cells, nerve roots, plexus, peripheral nerve and muscle (Chu-Andrews & Johnson 1986). They are manifestations of CL, reflecting the abnormally elevated sensitivity and reactivity of the muscle membrane to both ACh and the mechanical stimuli of the electromyography needle as it provokes depolarization, a result of the disinhibiting effect of denervation. Significantly, in addition to the spontaneous depolarizations that produce action potentials, ACh slowly depolarizes the supersensitive muscle membrane, inducing electromechanical coupling in which tension develops slowly without generating action potentials (Eyzaguirre & Fidone 1975).
Cannon and Rosenblueth’s original work was based on complete loss of motor innervation for supersensitivity to develop. Subsequently it became recognized that actual physical interruption and total denervation are not necessary: any injury or illness that impedes the flow of motor impulses for a period of time can rob the target organ of its excitatory input and cause supersensitivity in that structure and, significantly, in associated spinal reflexes (Loeser et al. 2001, Cangiano et al. 1977, Gilliat 1978). Supersensitive skeletal muscle fibers overreact to a wide variety of chemical and physical inputs, including stretch and pressure.
This process of nerve dysfunction with impaired or interrupted neural impulses and at times associated with partial denervation is not uncommon in adults, and is known as ‘neuropathy’, or ‘nerve-sickness’, literally. It is important to recognize that such a nerve still conducts nerve impulses, synthesizes and releases transmitted substances and in the case of motor nerves, evokes both muscle action potentials and muscle contraction. The causes of neuropathy are legion and include congenital, neoplasms, inflammatory, traumatic, vascular, toxic, metabolic, infectious, degenerative and idiopathic etiologies. Commonly recognized neuropathies include the peripheral sensory neuropathies associated with diabetes or alcoholism; however, the far more common cause of nerve dysfunction is trauma, including acute, sub-acute and chronic. Sciatica, a type of spondylotic traumatic compressive neuropathy, accounts for a relative incidence five times that of diabetic neuropathy in the USA (Bridges et al. 2001). Spondylosis is defined as the sub-acute or chronic (gradual, insidious) structural disintegration and morphologic alterations in the intervertebral disc and pathoanatomic changes in surrounding structures that leads to damage of the nerve roots and spinal nerves (Wilkinson 1971). Since the nerve roots and spinal nerves contain motor, sensory and autonomic fibers, it follows that the clinical manifestations of injury to them reflect the effects of neuropathy that develop to varying degrees in each of these three components, and will be discussed in subsequent sections of the chapter.
As noted earlier, in neuropathic skeletal muscle ACh slowly depolarizes the supersensitive muscle membrane, inducing electromechanical coupling of actin and myosin in which tension develops slowly without generating action potentials. As such, due to the extended time frame over which this occurs, no action potentials are seen on electromyography, and this shortening is called contracture rather than contraction (Eyzagguire & Fidone 1975). In addition, Gunn has proposed that radiculoneuropathic involvement of muscle spindle afferent fibers leads to hyperexcitability of the muscle spindle mechanism, potentiating the length-regulating feedback mechanism of the gamma loop and contributing to the development of these contractures (Gunn & Milbrandt 1977a). This mechanism may be amplified even further by sympathetic supersensitivity activating intrafusal fibers of the muscle spindle (Chu 1995). Dysfunction of this mechanism is sometimes referred to as the ‘facilitated segment’, ‘somatic dysfunction’, or the ‘osteopathic lesion’ (Korr 1975).
On physical exam these muscle contractures are palpable in the more superficial muscles, and are commonly referred to as ‘taut bands’, ‘ropy bands’ or ‘contraction knots’ (Baldry 2001). Deeper, non-palpable contractures are what Gunn terms ‘the silent lesion’ (Gunn 1996). Over time, when enough regions of the muscle develop contractures, the muscle’s overall resting length shortens, at which point the patient may become aware of decreased flexibility, noting for example the need to turn their upper body to check automotive traffic behind them, as the active range-of-motion in the cervical spine is diminished. As the process of spondylosis continues over time, and is aggravated by additional acute injuries, the model postulates that smaller diameter nerve fibers develop supersensitivity and myalgic hyperalgesia develops. The patient may still be otherwise asymptomatic, with the exception perhaps of complaints of ‘stiffness’, and surprised by the pain elicited by palpation of these tender contractures, or ‘latent TrPs’ (Baldry 2001). It is this morbid but generally symptom-free phase that Gunn terms ‘pre-spondylosis’ (Gunn 1980). Eventually, as both the muscle shortening compresses intramuscular type III and IV nociceptors and sensitization of small nociceptor fiber advances, the patient develops active TrPs and complains of spontaneous pain.
The combined sensory and autonomic effects of RNMP manifest as all manner of subjective complaints including paresthesias (including tingling, buzzing, vibration, pressure), dysesthesias including ‘pins and needles’, neuralgic pain (shooting-stabbing-lancinating-paroxysmal) and itching (Stellon 2002); complaints of stiffness and swelling are attributable to muscle shortening and neurogenic edema. Physical findings may include decreased range-of-motion, myalgic hyperalgesia with generation of referred pain and either spontaneous or elicited local fasciculation, also referred to as the ‘local twitch response’ (LTR). There may be cutaneous hypoesthesia, and allodynia, rather than anesthesia which is present in denervation.
As over time RNMP leads to both localized intramuscular contractures and shortening of the resting length of the muscle, the persistent increased mechanical tension on the musculotendinous attachments to bone leads to what previously was labeled tendonitis, implying an inflammatory etiology, now preferably called tendonopathy or tendonosis (Khan et al. 1999). These include most of the sub-acute and chronic tendonitis and bursitis syndromes (Achilles, extensor forearm, bicipital, rotator cuff, DeQuervain’s tenosynovitis, patella, gluteal) as well as such entities as iliotibial band syndrome, chondromalacia patellae, muscle tension headache, pyriformis syndrome, plantar fasciitis and temporomandibular joint. Many of these are thus seen as the effects of muscle shortening, which due to increased tension at the periosteum create pain as well as bone spurs according to Wolff’s Law, which describes the process of bone deposition in response to mechanical tensile forces. Thus extensor elbow tendonosis, or ‘tennis elbow’, is seen not as a local pathology but the ‘downstream’ effect of sub-acute and chronic C6–C7 radiculoneuropathic-myofascial pain that can sometimes be treated successfully by treatment to the cervical spine alone (Gunn and Milbrandt, 1976b).
The constellation of symptoms and signs noted above collectively constitute a picture and definition of what is called MPS, myofascial pain being a term popularized by Janet Travell, MD, who also ‘popularized the use of the term TrP’ (Baldry 2001). The pathognomonic feature of MPS is the TrP, the hallmark of which is hyperalgesia with referred pain, and which is ‘structurally…made up of a collection of dysfunctional motor endplates, juxtapositional contraction knots and neurovascular bundles with each containing blood vessels and contiguous sympathetic fibres; a motor axon and its nerve terminals; and sensory afferents attached to proprioceptors and nociceptors’ (Baldry 2001).
While Gunn’s model recognizes TrPs as most often found beneath motor points and the importance of directing treatment to these points (Gunn et al. 1980), the TrP is seen as just one of the many diverse manifestations of radiculoneuropathic-myofascial pain syndrome. It recognizes that needling can ‘occasionally actuate muscle to fasciculation: this is usually accompanied by near-instantaneous muscle relaxation’ (LTR), but also, that due to supersensitivity the entire surface of the neuropathic muscle may respond to needling. Penetration into almost any part of the muscle can lead to relaxation, but the most rewarding sites are at tender and painful points in muscle bands (Gunn, 1989b).
Gunn’s finding of a correlation between tender motor points and electromyography (EMG) evidence of partial denervation radiculopathy has been corroborated by Chu using a semi-quantitative motor unit action potential (MUAP) EMG technique (Chu 1995). While EMG abnormalities were found in a myotomal distribution correlating with clinical findings of MPS, Chu suggested that single-fiber EMG (SFEMG) may be more useful than conventional EMG in establishing the cause of abnormalities as ‘neurogenic, myogenic, or otherwise.’ The presence and severity of motor neuroaxonal degeneration correlating with TrPs and disease duration using SFEMG technique has recently been established by Chang, who has also found evidence of spinal accessory neuropathy in cervical MPS (Chang et al. 2008, 2011).
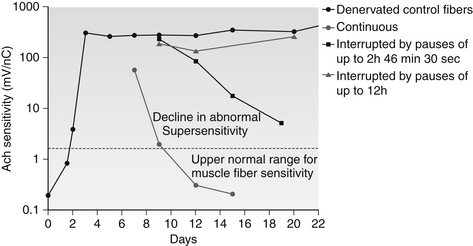
What then is the evidence that Gunn-IMS can reverse RNMP? Evidence that neuropathic supersensitivity can be reversed was demonstrated experimentally by Lomo, who showed that ACh supersensitivity in denervated animal skeletal muscle could be abolished by graded electrical stimuli (Theslef, 1976). Figure 14.1 shows how experimental denervation affects the sensitivity of a muscle membrane to ACh (bold line). Additionally, Figure 14.1 shows how this hypersensitivity returns toward normal after electrical stimulation, and does so more quickly with continuously applied stimuli. Gunn has proposed that the ‘current of injury’ (the measureable microcurrent associated with damage to a cell wall membrane) created by the minor muscle fiber trauma of needling provides an intrinsic source of electrical stimulation that facilitates reversal of neuropathic supersensitivity similar to that provided exogenously by Lomo (Gunn 1978).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree