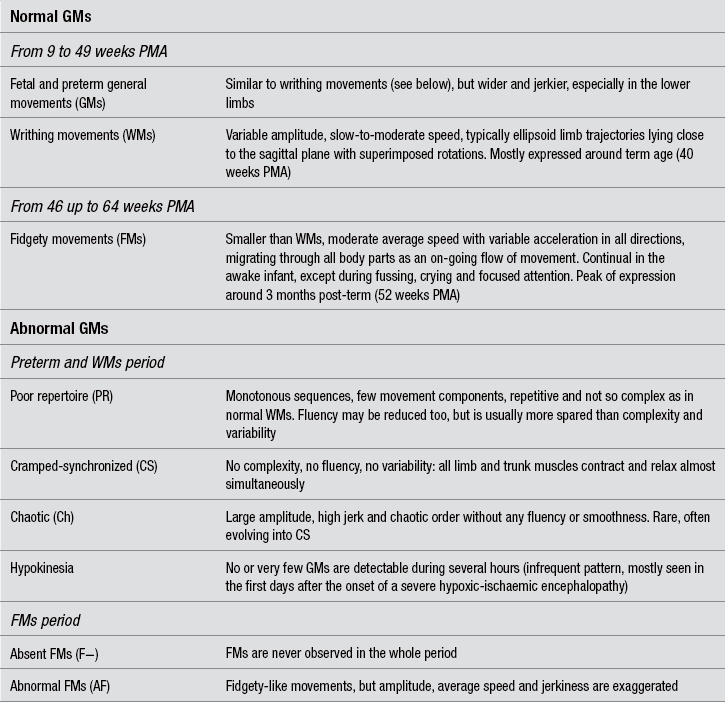
8 Techniques for the clinical assessment of the neonatal nervous system Prechtl’s method on the qualitative assessment of general movements Absent fidgety movements at 3–5 months is the most sensitive sign for CP prediction Cramped-synchronized general movements at preterm and term age are a very specific predictive sign for spastic CP The asymmetry of selective distal movements at 3 months predicts unilateral CP GM features that can predict dyskinetic CP Motor optimality scores and the prediction of CP severity Integrating GM assessment with traditional neurological examination and neuroimaging techniques The quality of care for the infants born with an extremely low birth weight, or with other conditions of high neurological risk, has achieved extraordinary progress in the last decades. Survival and quality of life have considerably increased for these children, but they still remain at risk for neurological damage, caused by perinatal infections, hypoxic-ischaemic damage, haemorrhagic insult, or by a combination of these factors. Negative outcome includes cerebral palsy (CP), intellectual disability, perceptual and sensory disorders, behavioural disorders and other. Although prognostic hints for a CP diagnosis are needed in the neonatal period, many textbooks and manuals still postpone the possibility of a diagnosis of CP to the end of a so-called ‘silent period’, lasting some weeks or even, for mild forms of CP, some months after term age. The possibility that neonatal neuroimaging can enable recognition of brain pathology that may lead to a diagnosis of CP is today largely accepted in terms of the type and timing of the brain lesions typical of the different forms of CP (Krägeloh-Mann, 2004), although it is questioned by some authors (O’Shea et al., 1998). A recent paper by de Vries et al. (2011) has unravelled the ‘myth’ that CP cannot be predicted by neonatal neuroimaging, when this technique is correctly applied. The authors show evidence from a number of papers that the non-ambulatory most severe CP can be predicted in the majority of the cases by a combination of sequential brain US and by MRI carried out at term age. Additional useful indices can be obtained by assessing the myelination of the PLIC (posterior limb of internal capsule) and diffusion-weighted imaging techniques. Moreover, there are interesting research directions that may further improve, by sophisticated MRI techniques, the prognostic value of neonatal neuroimaging in the less severe forms of CP. Some of the standard neurological examination methods, though standing as milestones of modern infant neurology, are still influenced by considerations drawn from adult neurology and experiments on animal models. For instance, Saint-Anne Dargassies (1977) developed a pioneering examination protocol based on the evaluation of active and passive tone. Other methods were then proposed in the following decades, including items for muscle tone, postural-motor milestones and, in some cases, behavioural aspects. The neurological examination protocol proposed in the same period by Prechtl (1977) (not to be confused with the method the same author has later proposed), has been standardized and validated only for the examination of infants at term. It includes the extremely important concept of behavioural states, but many of its items are still based on muscle tone and responses integrated at a low level in the CNS. Moreover, it is rather time-consuming and cannot be applied to preterm infants. The Neonatal Behavioural Assessment Scale (NBAS) is a technique developed by Brazelton (Brazelton and Nugent, 1995) for examining the behaviour of term infants during the first couple of months of age. Its conceptual basis is founded on the assumption that the newborn has active and specific responses to environmental stimulations, rather than passive behaviour. On the basis of NBAS, Als (1984) standardized a behavioural scale for preterm infants, the Assessment of Preterm Infant Behaviour (APIB), thought to be employed in neonatal intensive care units also for providing and monitoring individualized intervention programmes. These techniques are time-consuming and not easily applicable in clinical settings. Moreover, a high intra-individual day-to-day variability in the responses has been shown for the same test (Sameroff, 1978). Their main applications are in research and early intervention protocols. Currently, the most recently updated and extensively validated method for the neurological examination of preterm and full-term newborn infants is the Hammersmith Neonatal Neurological Examination (HNNE), first published by Dubowitz and Dubowitz (1981)and updated by Dubowitz et al. (1999). These authors adapted tests drawn from the previous works of Prechtl, Saint-Anne Dargassies and Brazelton, into a simplified and user-friendly pro forma, also including items based on the concepts of Prechtl and co-workers on spontaneous motor activity (see below). The items are organized in six sections: posture and tone; tone patterns; reflexes; movements; abnormal signs; and behaviour. Typical normal and abnormal patterns are extensively described in the manual (Dubowitz et al., 1999) and have proven easily recognizable and clinically useful for diagnosis and prognosis. For research purposes, an optimality score for full-term and preterm newborn infants was also calculated (Mercuri et al., 2003). On the basis of the neonatal examination, the same authors also developed a protocol for use after the neonatal period in infants up to 24 months of age: the Hammersmith Infant Neurological Examination (HINE) (Dubowitz et al., 1999), divided into three sections: one, non-age-dependent neurological items; a second providing a summary of motor milestones; and a third made of three simple behavioural items. Its prognostic value as to motor outcome has been found high both in preterm infants born before 31 weeks of gestation (Frisone et al., 2002) and in term infants with hypoxic-ischemic encephalopathy (Haataja et al., 2001). Although both HNNE and HINE have been tested in several clinical and research settings, they also present some limitations. Most items are still correlated with muscle tone and reflexes and the distinction between normal and abnormal patterns may turn out, to some extent, rigid and schematic, hardly comprising the whole complexity of the infant’s repertoire. Moreover, while several studies have reported statistically significant correlations between clinical findings, and mid- and long-term outcome, some others have reported a relevant number of false-positive and false-negative results, especially among preterm infants (see Volpe, 2008, for a review of follow-up studies). The basic requirements for an ideal method of neurological assessment for newborn infants were outlined by Heinz Prechtl as follows: it had to be non-invasive, non-time-consuming, and highly sensitive to variations of the age-specific functional repertoire (Prechtl, 1990). None of the traditional protocols of neonatal neurological assessment completely matched these criteria. The observation and categorization of all spontaneous movements in the first months of life led Prechtl and his co-workers to the identification of several normal and abnormal motor patterns. Among others, the so-called ‘general movements’ (GMs) were identified as on-going global movements involving all body parts and appeared particularly suitable for assessment (Prechtl, 1990, 2001). This gave birth to Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term and Young Infants (see the published handbook: Einspieler et al., 2004). Though based on global and qualitative judgement, GM assessment has proven a very sound method. Its validity and reliability have been extensively evaluated in a number of studies, mainly dealing with the prediction of CP (Cioni et al., 2000; Ferrari et al., 2002; Prechtl, 1997), but also with that of minor neurological disorders (Bruggink et al., 2008; Einspieler et al., 2007; Groen et al. 2005), Rett syndrome (Einspieler et al., 2005a,b), cognitive development (Bruggink et al., 2010) and autistic spectrum disorders (Phagava et al., 2008). Normal GMs involve the whole body in a complex sequence of arm, leg, neck and trunk movements. They wax and wane in intensity, force and speed, and have a gradual beginning and end. Rotations along the axis of the limbs and slight changes in the direction of movement make them fluent and elegant and create the impression of complexity and variability. GMs appear as early as nine to 12 weeks postmenstrual age (PMA) and continue after birth without substantially changing their form until 46 to 49 weeks PMA, irrespective of when birth occurs. At 46 to 49 weeks PMA, a major change in form sets in, being fulfilled around 3 months post-term, when the so-called fidgety movements (FMs) appear (Hadders-Algra and Prechtl, 1992; Prechtl, 1990). From 5 to 6 months post-term, GMs fade out, while new, voluntary motor patterns emerge. Though complex and variable by definition, GMs can be classified into a limited number of recognizable patterns, related to PMA and either normal or abnormal. Normal GMs are briefly reported in Table 8.1. As already stated, in the FMs period various other motor patterns gradually emerge and mingle with GMs, thus building up the so-called ‘associated motor repertoire’, whose richness and age-adequacy have been related to the optimality of later motor co-ordination (Bruggink et al., 2008) and cognitive functions (Bruggink et al., 2010). GMs of infants with cerebral impairment lack complexity, fluency and/or variability. Abnormal GM patterns can be sorted into two groups depending on whether they are observed before or after the onset of FMs, i.e., in the preterm/writhing movement period or in the FMs period. The description of these patterns is also reported in Table 8.1. The global visual perception of movement quality (Gestalt perception) has proven a powerful and reliable instrument to recognize normal and abnormal GMs, but only if scorers are properly trained and the technique is carefully applied. A thorough description of the standardized assessment procedure can be found in the GMs handbook (Einspieler et al., 2004). Notably, the standard GM assessment is performed off-line on selected video recordings, but it has also proven reliable (especially in the FMs period) when performed live, as part of the routine neurological examination. The methodology of Prechtl’s GM assessment has evolved to today’s standardized and highly reliable form through several years. Concerns about its possible biases (e.g., because of poor validation in non-European countries) may still arise (see, for instance, Darsaklis et al., 2011), but they can be overestimated if earlier studies are considered together with newer ones, or if results obtained in different clinical populations (e.g. high-risk and low-risk babies) are mixed-up. Recently, the most predictive features of GMs have been definitely pointed out and their clinical role extensively reviewed, as summarized in the following paragraphs, especially in relation to prediction of CP. In 1997, Heinz Prechtl and associates carried out the most important study to date on the predictive value of GM assessment, indicating it to be a reliable and valid tool for distinguishing between infants who are at significant risk of developing CP and infants who are not (Precthl et al., 1997). The findings were based on a longitudinal study on 130 infants who represented the whole spectrum of perinatal brain ultrasound findings. Central to the study were the age-specific FMs, i.e., normal FMs observed at least once between 3 and 5 months post-term age. Ninety-six per cent of the infants with normal FMs (n=70) had a normal neurological outcome. Abnormal quality or total absence of FMs were followed by neurological abnormalities (most of them CP) in 95% of the 60 infants. Specificity and sensitivity of the assessment of FMs (96% and 95%, respectively) were higher than those of cranial ultrasound (83% and 80%, respectively). Since then, various groups have emphasized the significance of FMs for the early prediction of CP. Burger and Louw (2009) reviewed 15 studies on the predictive value of FMs and reported a sensitivity>91% and a specificity>81%. So far, the largest sample recruited in a longitudinal study has been of 903 children, which yielded a sensitivity of 98% and a specificity of 94% (Romeo et al., 2009). As already mentioned, GM assessment is based on global and qualitative judgement, but has proven highly reliable and consistent, both inter- and intra-subjectively, especially in the FMs period. Its high level of objectivity has been documented by an inter-scorer agreement ranging from 89% to 93%, and by an average kappa of 0.88, both obtained in a total of 15 studies (Einspieler and Prechtl, 2005; Fjørtoft et al., 2009). Such high values can be achieved after a few days of extensive training (Valentin et al., 2005). A high intra-individual consistency of GM quality was demonstrated by kappa values from 0.90 to 0.96 (Mutlu et al., 2008). Due to the utmost importance of FMs for prognosis, recent research has struggled to provide automated and objective methods for their identification. A computer-based video analysis technique has been recently developed by Adde et al. (2009, 2010), yielding a sensitivity of 85% and a specificity of 88% for CP prediction. The mere absence of FMs, however, has never been found specific for a particular CP subtype, nor it can predict CP severity. This fact indicates that several neural structures, at least the corticospinal fibres, the basal ganglia and the cerebellum, need to be intact to generate normal FMs. The latter are thought to be a necessary step for an optimal calibration of the sensory-motor system (Prechtl et al., 1997). Interestingly enough, normal FMs are also absent in infants with some genetic disorders. FMs are thus very sensitive for prognosis, while other motor features, combined with the absence of FMs, have proven useful to predict CP type and severity.
Early diagnosis and prognosis in cerebral palsy
Techniques for the clinical assessment of the neonatal nervous system
Prechtl’s method on the qualitative assessment of general movements
Absent fidgety movements at 3–5 months is the most sensitive sign for CP prediction
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine