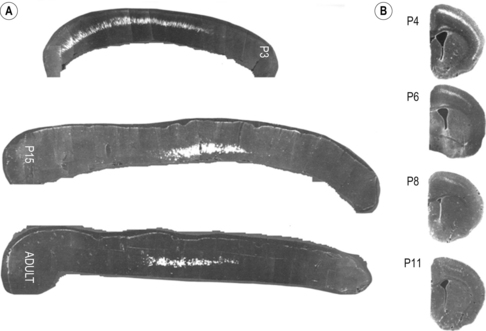
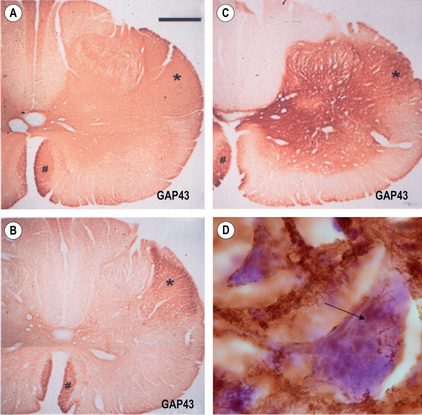
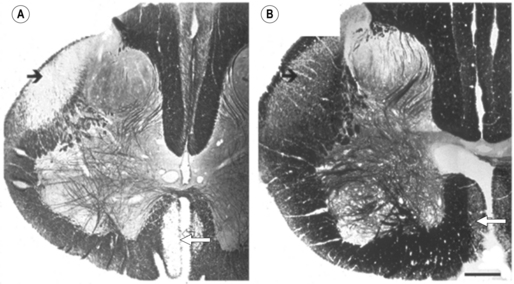
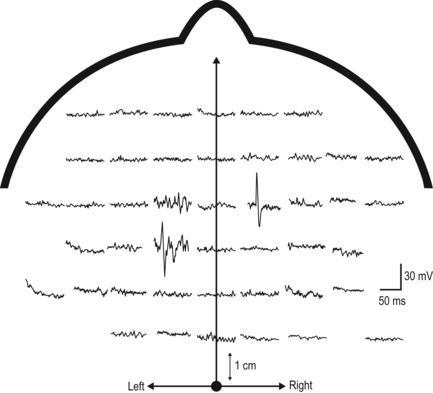
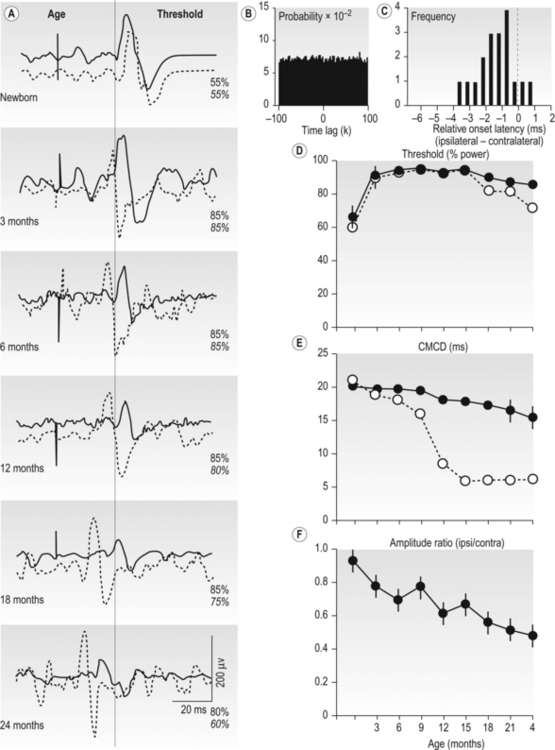
2 Man is unrivalled in achieving complex language, remarkably skilled and flexible manual dexterity and adept bipedal gait, abilities that are dependent upon highly proficient neural control of motoneurons and muscles. The performance of skilled movements has been shown to rely upon the integrity of the corticospinal system and specifically on direct monosynaptic input from the motor cortex to spinal α-motoneurons (Armand et al., 1996; Bortoff and Strick, 1993; Kuypers, 1962, 1981; Lawrence and Hopkins, 1976; Porter and Lemon, 1993). In sub-human primates, direct corticomotoneuronal projections are largely restricted to the motoneurons of muscles controlling movements of the arm, hand, foot and tail and their density is related to the degree of skill achieved (Bortoff and Strick, 1993; Heffner and Masterton, 1983; Kuypers, 1981; Phillips and Porter , 1977; Porter and Lemon, 1993). Man is unique in possessing direct corticomotoneuronal projections to all motor nuclei so far investigated (Porter and Lemon, 1993), implying a greatly expanded role for the corticomotoneuronal system. In humans, skilled voluntary control of movements develops postnatally, with the initial rapid emergence of motor skills over the first 18–24 postnatal months. These skills are subsequently refined over a prolonged period, until early adulthood (Forssberg et al., 1991; Muetzel et al., 2008). While the maturation of many systems, including the visuomotor and sensorimotor systems, and the cognitive aspects of movement control such as selective attention and executive control will determine the expression of developing motor skills and the eventual level of dexterity achieved (Martins et al., 2012), these behavioural changes also undoubtedly reflect the prolonged maturation throughout childhood and adolescence of the corticospinal system. If damage to the corticospinal system occurs in adulthood, great difficulty in learning new or relearning former sequences of skilled movements occurs (Brodal, 1973), emphasizing the role of the corticospinal system in the acquisition and maintenance of skill. When lesions occur in the perinatal period, not only is learning of skilled movements severely impaired but also such lesions can result in the development of cerebral palsy. In the immediate period after the lesion, however, babies who will develop cerebral palsy display only subtle, if any, abnormalities of movement control (Bouza et al., 1994; Nelson and Ellenberg, 1979; Prechtl et al., 1997). There follows over many months and years, the progressive development of a movement disorder, associated with significant secondary disruption of corticospinal and spinal motor centre development (Eyre et al., 2007; Leonard et al., 1991; Myklebust et al., 1982; O’Sullivan et al., 1998). This implies a further role for the corticomotoneuronal system in man, activity-dependent regulation of the development of the sensorimotor cortex, spinal motor centres and their connectivity. Older concepts of developmental plasticity focused on the protective effect of a young age at the time of the brain damage (Kennard, 1936). In these views, a younger rather than an older age at onset was thought to produce fewer or less severe symptoms and a more rapid recovery. It is now clear that the specific effects of early brain damage produce complex and often severe patterns of impairment, which are different from those observed following lesions in the adult brain. Furthermore, although an understanding of the nature of neural plasticity in response to damage is critical to those attempting to augment recovery from neurological insults, it is misleading to treat the underlying mechanisms as self-reparative. The appreciation that these mechanisms evolved as an expedient for fine-tuning neurological circuitry during normal development by taking advantage of contextual information, and may only be available for response to damage as an incidental side effect, can help to focus on how best to augment the desirable and avoid any undesirable effects. Seminal studies in the early 1960s by Wiesel and Hubel (1965) on the organization of ocular dominance columns signified the starting point of extensive basic research on developmental brain plasticity. The investigation of neuroplasticity has expanded rapidly over the past 10 years and has uncovered the remarkable capacity of the developing brain to be shaped by activity and environmental input. Knowledge of the time course and processes of normal development is essential both for a better understanding of current rehabilitation treatments and for the design of new strategies for the treatment of children who sustain brain damage early in life. The development of the corticospinal system has been studied most extensively in the rat. In the neonatal rat the corticospinal projection originates from the whole neocortex, including the visual cortex (O’Leary et al., 1992; Stanfield and O’Leary, 1985) (Fig. 2.1). Corticospinal projections in several mammalian species also have transient ipsilateral projections early in development that are predominantly eliminated when maturity is reached (O’Leary et al., 1992). Massive axonal withdrawal, coupled with modest corticospinal cell death, leads to complete elimination of the corticospinal projection from inappropriate regions of the cortex, a reduction in the number of corticospinal axons projecting from the primary sensorimotor cortex and almost complete withdrawal of the ipsilateral projection (Joosten et al., 1992; Oudega et al., 1994). Figure 2.1 (A) Sagittal sections through the cortex of a rat aged 3 postnatal days (P3), 15 postnatal days (P15) and in adulthood showing labelled cells following an injection of Fast blue at the high cervical level. (B) Coronal sections through the anterior parietal cortex in rats aged from 4 to 11 postnatal days (P4–P11) showing labelled cortical cells following injection of horseradish peroxidase into the high cervical spinal cord. [(A) Adapted from Schreyer D, Jones E. Growth and target finding by axons of the corticospinal tract in prenatal and postnatal rats. Neuroscience 1982;7:1837−1853, with permission from Elsevier. (B) Adapted from Bates C, Killackey H. The emergence of a discretely distributed pattern of corticospinal projection neurons. Brain Res. 1984;13:265−273, with permission from Elsevier.] Substantial lesions of the sensorimotor cortex or corticospinal tract in sub-primate mammals early in postnatal life lead to hypertrophy of the undamaged motor cortex and corticospinal projection, which has an increased number of corticospinal axons (Hicks and D’Amato, 1970, 1977; Huttenlocher and Raichelson, 1989; Jansen and Low, 1996; Rouiller et al., 1991; Uematsu et al., 1996). These changes are associated with maintenance of an increased ipsilateral corticospinal projection from the undamaged hemisphere. The cells of origin of the aberrant ipsilateral axons are more widely distributed and distinct from the cells of origin of the crossed or contralateral corticospinal projection (Huttenlocher and Raichelson, 1989; Jansen and Low, 1996; O’Leary et al., 1992; Reinoso and Castro, 1989). There is no evidence for double labelling of corticospinal neurons in neonatally hemispherectomized animals that in adulthood had spinal cord injection of fluorescent tracers (Reinoso and Castro, 1989). Thus the ipsilaterally projecting corticospinal axons from the undamaged cortex do not arise solely as branches of the contralateral corticospinal projection, but arise also from neurons that extend axons into the ipsilateral spinal cord during development, and whose axons would normally be withdrawn. The distribution of aberrant ipsilateral axons within the spinal grey matter resembles that of the contralateral corticospinal projection (Barth and Stanfield, 1990; McClung and Castro, 1975)and synaptic contacts have been demonstrated (Leong, 1976; McClung and Castro, 1975). Ipsilateral forelimb movements are observed following stimulation of the intact cortex at abnormally low current thresholds, which are abolished by medullary pyramidotomy (Kartje-Tillotson et al., 1985, 1987). It has been proposed that corticospinal innervation in primates may not be governed by the same processes or have the degree of plasticity described in sub-primate mammals (Armand et al., 1997; O’Leary et al., 1992). However in the macaque monkey a halving of the area of the cerebral cortex from which corticospinal axons originate has been demonstrated during the first eight postnatal months, when brain volume overall increases by more than 30%. These changes are associated with a threefold reduction in the number of retrogradely labelled cortical neurons, providing convincing evidence for an initially exuberant corticospinal projection and significant corticospinal axonal withdrawal (Galea and Darian-Smith, 1995). Although Kuypers (1962) and Armand et al. (1997) found no evidence for postnatal reduction in corticospinal synapses in the cervical spinal cord during the same period in macaque monkeys, this may reflect the methodology employed. Both studies used anterograde labelling of axons projecting from the hand area of the primary motor cortex. Such focal anterograde labelling would not detect projection and withdrawal of corticospinal axons and synapses from other areas of the cortex, including other areas of the motor cortex. It is from these other areas that the majority of supernumerary axons arise both in sub-primate mammals (Stanfield et al., 1982) and also in the macaque monkey (Galea and Darian-Smith, 1995). Furthermore, elimination of supernumerary synapses occurs in conjunction with the proliferation of synapses from the subset of axons that are maintained. It is the subset of axons that are maintained which would be labelled by anterograde tracers. Net increases in synaptic density have been observed during significant axonal withdrawal in other primate systems (LaMantia and Rakic, 1990, 1994). Studies in monkeys have failed to reveal plasticity of corticospinal tract development following lesions; however, no study has yet replicated the circumstances in which plasticity has been demonstrated in sub-primate mammals and in humans (see below). Passingham et al. (1983) performed lesions too late, with all but one being between postnatal days 23 and 89. Rouiller et al. (1991) made very focal lesions in the motor cortex and demonstrated substantial reorganization of the surrounding motor cortex on the same side as the lesion. Reorganization of the ipsilateral projection from the undamaged hemisphere in sub-primate mammals has only been observed following large lesions such as ablation or extensive infarction of the motor cortex, or following pyramidotomy. Finally, Galea and Darian Smith (1997) performed a hemisection procedure of the cervical spinal cord in a monkey with the lesion located below the pyramidal decussation and involved the projections from both hemispheres. The detailed study did, however, exclude significant branching of corticospinal axons at spinal levels in response to early lesions of the corticospinal tract. By eight weeks post-conceptional age (PCA) corticospinal axons reach the medulla (Humphrey, 1960; O’Rahily and Müller, 1994) and decussation occurs at about 15 weeks PCA. Corticospinal axons invade the axon tracts of the spinal cord from 17 to 29 weeks PCA (Altman and Bayer, 2001) and reach the lower cervical spinal cord by 24 weeks PCA (Fig. 2.2). Corticospinal innervation of the spinal motoneuronal pools can be demonstrated by at least 31 weeks PCA (Fig. 2.2C). There follows progressive innervation of the grey matter so that there is extensive innervation of spinal neurons, including motoneurons, prior to birth (Eyre, 2005; Eyre et al., 2000a, 2002). By 40 weeks PCA, corticospinal axons have begun to express neurofilaments and to undergo myelination (Fig. 2.3). Figure 2.2 Horizontal sections of human spinal cord C5-6. (A) 24 weeks PCA. GAP43 immunoreactivity is widespread in white and grey matter. (B) 27 weeks PCA. Corticospinal tracts are the only major axon tracts expressing GAP43 from which weaker immunoreactivity extends into the intermediate grey matter. (C) 31 weeks PCA. Immunoreactivity is also now intense in the intermediate grey matter and present in motoneuronal pools and dorsal horn. (D) 35 weeks PCA. Section counterstained with cresyl violet. #, Nissl-stained motoneuronal cell body; arrow, GAP43 expressing varicose axons. Motoneuron cell bodies are closely apposed by GAP43 immunoreactive varicose axons. (A–C), Scale bar, 500 μm. Stars mark the lateral and the anterior corticospinal tracts. (D) Scale bar, 20 μm. [From Eyre J, Miller S, Clowry G, Conway E, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain 2000;123:51–64, reprinted by permission of Oxford University Press.] Figure 2.3 Onset of myelination of the human corticospinal tract in the lower cervical spinal cord. Sections of human spinal cord, level C5,6. (A) 24 weeks PCA and (B) 40 weeks PCA stained for myelin. The black arrows mark the lateral corticospinal tract and the white arrows, the anterior corticospinal tract, both of which stand out clearly because of their lack of myelination relative to most other white matter tracts. From our studies of GAP43 immunoreactivity (Fig. 2.2), we know that corticospinal axons are present at 24 weeks PCA and it appears at this age that only occasional fibres within the tract may be myelinated. By birth, myelination is clearly underway although far from complete. [Reprinted from Eyre JA, Miller S, Clowry GJ. The development of the corticospinal tract in humans. In: Pascual-Leone A, Davey G, Rothwell J, Wasserman EM, editors. Handbook of Transcranial Magnetic Stimulation. London: Arnold, 2002, p. 235–49, with permission of Hodder UK Ltd.] Detailed neurophysiological studies (Eyre et al., 2000a; Szelenyi et al., 2003) provided compelling evidence for the ability of the corticospinal axons in the term baby to conduct a coherent volley and for the prenatal establishment of functional, corticospinal synapses with both α-motoneurons and spinal interneurons influencing motoneuronal pool excitability. This early corticospinal innervation provides the capacity for activity in the corticospinal system to be intimately involved in shaping the development of the motor cortex, spinal motor centres and their connectivity through the corticospinal tract (Basu et al., 2010; Eyre, 2004, 2005, 2007; Eyre et al., 2000a, 2001), reflecting the uniquely dominant role of the corticomotoneuronal system in human movement control. In maturity, cortical neurons projecting to specific subcortical targets are restricted to specialized areas of the neocortex. Thus, corticospinal neurons are largely limited to the primary sensorimotor cortex, whilst corticotectal neurons projecting to the superior colliculus are found primarily in the visual cortex. However, studies in sub-primate mammals reveal that neurons with subcortical projections initially project non-specifically to all subcortical target areas via axonal collateral branches. The mature, focused pattern is subsequently achieved by a selective pattern of withdrawal of the axonal collateral branches of neurons within each specialized projection area of the cerebral cortex (O’Leary and Koester, 1993; O’Leary and Stanfield, 1985; Stanfield et al., 1982). This process is activity dependent, since rat pups exposed to the NMDA receptor antagonist MK-801 during the period of axonal refinement maintain a significantly increased corticospinal projection, which includes aberrant corticospinal projections from the occipital cortices (O’Donoghue et al., 1993). Thyroid hormone may also have a role in axonal withdrawal since pre- and postnatally hypothyroid rats also maintain inappropriate corticospinal projections from the occipital cortex (Li et al., 1995). Furthermore, if a portion of fetal rat occipital cortex is transplanted to the frontal region in newborn hosts, neurons in the transplant will maintain a corticospinal projection but withdraw their corticotectal projection. Conversely, fetal frontal cortex transplanted to the occipital region will withdraw corticospinal projections whilst maintaining projections to the tectum (O’Leary and Koester, 1993). Finally, occipital projections to the spinal cord persist in the newborn rat if a fetal tectum is transplanted to the spinal cord at birth and at the same time the host tectum is removed (Sharkey et al., 1986). In longitudinal and cross-sectional studies of normal babies and children, neurophysiological findings are consistent with significant withdrawal of corticospinal axons occurring over the first 24 postnatal months (Basu et al., 2010; Eyre, 2007; Eyre et al., 2001, 2007). Axonal withdrawal has also been observed directly in studies of developing sub-human primates (Galea and Darian-Smith, 1995). We have demonstrated significant bilateral innervation of spinal motoneuronal pools from each motor cortex in neonates. Thus, focal transcranial magnetic stimulation (TMS) of the motor cortex evokes responses in ipsilateral and contralateral muscles that have similar thresholds and amplitudes but shorter onset latencies ipsilaterally, consistent with the shorter ipsilateral pathway length (Eyre et al., 2001, 2007) (Figs 2.4 and 2.5). Rapid differential development of the ipsilateral and contralateral projections was observed so that the responses evoked at 2 years postnatal age in ipsilateral muscles are less frequent, significantly smaller, and have longer onset latencies and had higher thresholds than responses in contralateral muscles (Fig. 2.5). This differential development of the ipsilateral responses is consistent with a greater withdrawal of ipsilateral corticospinal projections than contralateral, as has been observed during development of the corticospinal tract in animals (Joosten et al., 1992; O’Leary et al., 1992). The small and late ipsilateral responses observed in older children and adults are consistent with the persistence of a small ipsilateral corticospinal projection, with slower conducting axons than contralateral projections. This conclusion is supported by anatomical studies in man and monkeys that demonstrate in maturity the corticospinal tract has approximately 8–15% of uncrossed axons (Armand et al., 1997; Galea and Darian-Smith, 1994; Nathan et al., 1990). These ipsilaterally projecting axons have been shown in man and in sub-human primates to arise from similar areas of the cortex as the contralaterally projecting axons and to have a significant overlap in their areas of termination with the contralateral corticospinal projection from the other hemisphere both within spinal grey matter and motoneuronal pools (Galea and Darian-Smith, 1994; Lacroix et al., 2004; Liu and Chambers, 1964; Nathan et al., 1990). Figure 2.4 Mapping of the origin of responses evoked in right biceps brachii using transcranial magnetic stimulation (TMS) and a focal figure-of-eight coil. The coil was positioned using a 1 cm×1 cm latex grid placed on the scalp. The subject was 3 weeks old. The filled circle marks the vertex. The electromyography traces are recorded in right biceps brachii. TMS was applied at the beginning of each trace. Responses were obtained in right biceps brachii following stimulation of both the ipsilateral and contralateral cortex. Figure 2.5 (A) Serial ipsilateral and contralateral responses recorded in the electromyography (EMG) of biceps following transcranial magnetic stimulation (TMS) of the left cortex in the same normal subject at increasing ages. The continuous line traces are from ipsilateral (left) biceps and dashed line traces are from contralateral (right) biceps. The stimulus artefact marks the application of TMS. The vertical line indicates the onset of the ipsilateral response when the subject was newborn. Thresholds for the responses are recorded on the right above the traces. Those in italics are for contralateral responses. (B) Cross-correlogram of multi-unit EMGs from contracting right and left biceps in the same newborn subject illustrated in (A) demonstrating no evidence for common drive to the motoneuronal pools. (C) The relative onset latencies for the ipsilateral and contralateral responses in the 18 neonates studied, calculated by subtracting the onset of the ipsilateral response from that of the contralateral. These data demonstrate the significantly shorter onset latency of ipsilateral responses in the newborn period. (D–F) Longitudinal data from nine subjects, including the subject illustrated in (A) studied at three-monthly intervals. Filled symbols and continuous lines represent data from ipsilateral and open symbols and dashed lines from contralateral responses. The symbols represent the mean and the vertical lines the 95% confidence limits for mean. Threshold was measured as the per cent of maximum stimulator output. CMCD is the central conduction delay within the corticospinal tract. The amplitude ratio was calculated by dividing the peak-to-peak amplitude of the ipsilateral responses by that of the contralateral. The horizontal dashed line in (D) indicates a ratio of one where responses are of equal size. These data demonstrate differential development of ipsilateral and contralateral responses evoked by TMS so that by 15 months ipsilateral responses have significantly higher thresholds, longer CMCDs and smaller amplitudes compared to contralateral responses. [From Eyre J, Taylor J, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology 2001;57:1543–1554, with permission of Wolters Kluwer Health.]
Corticospinal tract development and activity-dependent plasticity
Corticospinal tract development and plasticity in sub-primate mammals
Corticospinal development and plasticity in sub-human primates
Corticospinal development in humans
Shaping of the efferent axonal projection from the neocortex
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Corticospinal tract development and activity-dependent plasticity





