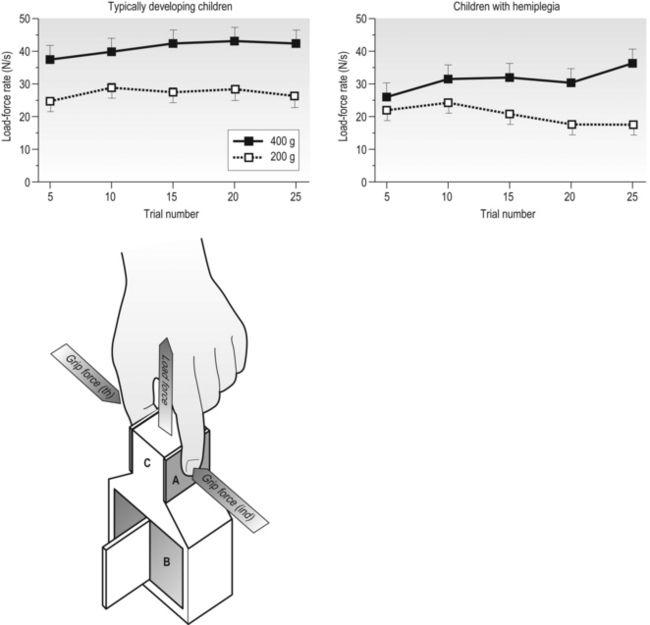
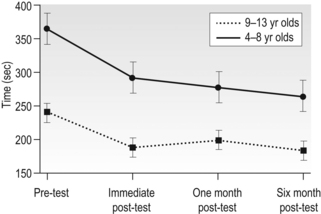
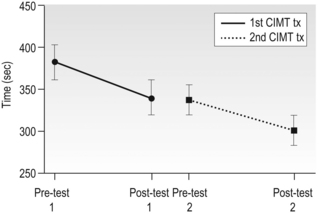
14 Unilateral cerebral palsy (CP), characterized by motor impairments mainly lateralized to one side, is among the most common subtypes of cerebral palsy, accounting for 30–40% of new cases (Himmelman et al., 2005). Through much of the last century the motor impairments, especially in the upper extremity (UE), were thought to be static with little potential for rehabilitation. Thus, rehabilitation efforts largely focused on minimizing impairments (e.g., reducing spasticity, preventing contractures). In fact, as recent as two decades ago, studies suggested that individuals with CP could reduce unwanted motor activity or spasticity with visual tracking or biofeedback, but that they had little ability to learn appropriate motor commands for skilled behaviours (Neilson et al., 1990; O’Dwyer and Neilson, 1988). Our own initial work studying prehensile force control reinforced this view, suggesting that children with CP retain infantile co-ordination strategies (Eliasson et al., 1991). However, subsequent research provided two separate lines of evidence that these impairments are actually not static. First, developmental studies of children with CP have shown that motor function does develop. For example, as children with CP get older, global motor function has been shown to improve (Rosenbaum et al., 2002). Development of hand function also occurs. For example, the longitudinal development of bimanual UE use in children with unilateral CP was recently studied (Holmefur et al., 2010). Children were followed for more than four years with the assisting hand assessment (AHA), a Rasch-based measure that describes how effectively the affected UE is used as a non-dominant assist during bimanual activities (Krumlinde-Sundholm and Eliasson, 2003; Krumlinde-Sundholm et al., 2007). Bimanual proficiency was found to improve during the course of development, but the rate of development and the time point of subsequent plateau depended on the initial level at 18 months of age. Specifically, children with unilateral CP with better bimanual function in early childhood developed bimanual skills and reached their plateau more quickly than children with worse initial bimanual function. It is interesting to note that the development of bimanual UE use differs from that of the lower extremity in CP (Rosenbaum et al., 2002), where children with milder impairments reach their limit later. In a separate 13-year follow-up study in children with CP starting at the age of 6–8 years, improvements in hand function were also seen with age (Eliasson et al., 2006). Specifically, the time to complete items on the Jebsen–Taylor Test of Hand Function (JTTHF) (Jebsen et al., 1969) improved in all children, and grip force co-ordination during manipulation improved over the 13-year period. Thus, motor functions of both upper and lower limbs do develop, although these two general types of skills seem to develop and plateau differently. A second line of evidence that motor function is not static in CP comes from studies on the effects of extended practice that have demonstrated that motor performance does improve with practice (Jarus and Gutman, 2001). In one study, children with CP were asked to repeatedly lift an object of a given weight 25 times (Gordon and Duff, 1999). Even though the learning of object properties used for force scaling was considerably slower than in typically developing children, impairments in manipulative capabilities and force regulation during object handling were partially ameliorated with this extended practice. This can be seen in Figure 14.1, which shows the load-force rate as a function of practice during consecutive lifts of objects weighing 200 g and 400 g. Unlike the typically developing children who show higher rates of force increase for the heavier object on the fifth trial, the rates of force increase are similar for the two weights at the same time point. However, after extended practice (20 to 25 lifts), higher rates of force increase are seen for the heavier object. This suggests that the initial impaired performance, at least in part, may be due to lack of use of the more affected UE and that there is residual motor capacity. In fact, this suggests that many impairments in motor control previously documented may be due to the fact that insufficient practice was provided (i.e., investigators were looking at the early stages of motor learning rather than motor control processes). Similarly, in-hand manipulation (Eliasson et al., 2003) and postural control (Shumway-Cook et al., 2003) have been found to improve with practice. These findings suggest that intensive practice may provide a window of opportunity for improvement. It is increasingly being recognized that reduction of spasticity alone (i.e., by the use of botulinum toxin) does not improve function (Rameckers et al., 2009), and that motor learning-based treatments and exercise provided with sufficient intensity have the potential to improve motor function in CP (Ahl et al., 2005; Gorter et al., 2009; Ketelaar et al., 2001; Verschuren et al., 2009). As in adults (Wu et al., 2000), children with CP may benefit more from practice with concrete tasks (van der Weel et al., 1991). For children with CP, experiences can be made concrete by using objects that are irresistible to pick up, i.e., the concrete component is provided by the environmental organization tied to instructions. Motor learning in this case may be enhanced by ensuring the task and object provide feedback on the effects of performance, as well as feedback from the therapist and higher-level cognitive strategies to achieve better performance levels (Gordon and Magill, 2012; Thorpe and Valvano, 2002). Figure 14.1 Mean±standard error of the mean (SEM) load-force rates for successive lifts with 200 g and 400 g objects as a function of trials for typically developing children (n=15) and children with hemiplegia (n=15). Note the large differences in the load-force rates for the control children during all blocks and small initial differences which increase across blocks for the children with hemiplegia. (A, forces transducers; B, exchangeable weight; C, position sensor) [Modified from Gordon AM, Duff SV. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. I: anticipatory scaling. Dev Med Child Neurol. 1999;41(3):166–175, with permission from John Wiley and Sons.] It is important to note that some methods of optimizing learning may not be applicable for those children with potential higher-level cognitive and proprioceptive impairments. Slower feedback processing even in typically developing children can result in information overload, which could interfere with learning. Thus, one would imagine they would benefit from intermittent feedback. However, such reduced feedback has been found to be less beneficial than constant feedback after every trial in children (Sullivan et al., 2008). Children, especially those with impaired sensory processing, may require increased feedback to update internal representations. Gradual reduction of feedback may be detrimental to learning in children (Sullivan et al., 2008). Feedback may therefore need to be withdrawn more gradually. Despite potential differences in motor learning strategies and capabilities, developmental studies and studies of motor learning in children with CP contradict traditional clinical assumptions that motor impairments in CP are static, and instead indicate that UE performance in children with CP may improve with practice and development. More importantly, these findings imply that hand function in particular may well be amenable to treatment, in particular with intensive practice. So what are the best models for delivery of intensive practice schedules? Unlike neuromuscular re-education treatments that traditionally focused on impairment-level disablement, task-oriented training focuses on the effectiveness and efficiency of motor performance in specific actions rather than on correction of movement patterns or prevention of compensations. Activity limitations are an important aspect of CP (Bax et al., 2005). Task-oriented training can be considered a motor learning (or goal-directed) approach to rehabilitation (Carr and Shepherd, 1989; Trombly, 1995; Winstein and Wolf, 2009) (see Chapters 1 and 11). This method of treatment is based on integrated models of motor learning and motor control and behavioural neuroscience. Focus is on participation and skill acquisition, and involves targeted physical and mental activity. An important component is the active problem solving described earlier. The associated behavioural demands of the tasks and motor skill training may result in cortical reorganization (Plautz et al., 2000) underlying concurrent functional outcomes. For optimal efficacy, the training must be challenging, with progressively increasing behavioural demands, and involve active participation. Skilled training in animals shows increased plasticity of UE cortical representations, whereas unskilled training did not (Kleim et al., 1998). At least beyond a certain point, time on task may be less important than what is practiced. Although evidence for functional and task-oriented training is accumulating, evidence for other treatments such as neurodevelopmental therapy (NDT) is more limited. An evidence report for the American Academy of Cerebral Palsy and Developmental Medicine concluded: ‘there was not consistent high-level evidence that NDT improved motoric responses, slowed or prevented contractures, or that it facilitated more normal motor development or functional motor activities’ (Butler and Darrah, 2001). There was a strong rationale from motor learning and neuroscientific studies underlying the application of intensive practice-based models to human UE rehabilitation (see, for example, Taub and Shee, 1980, Tower, 1940). Early attempts included ‘forced use’ in adult stroke patients, whereby the less-affected UE was restrained to passively induce practice of the more affected UE (Wolf et al., 1989). Subsequent attempts incorporated principles of psychology (shaping, whereby movements are reinforced using successive approximations) and motor learning to elicit active practice of the more affected UE, evolving to what is now known as ‘constraint-induced movement therapy’ (Taub and Wolf, 1997). Constraint-induced movement therapy (CIMT) has been studied extensively in adult hemiparetic stroke patients, where there is strong evidence of efficacy (see, for example, Wolf et al., 2006, 2008). CIMT has not been studied in the paediatric population nearly to the same extent that it has in adults with unilateral CP. Nevertheless, since our initial case study more than a decade ago (Charles et al., 2001), there have been nearly 70 studies of CIMT, including 27 randomized controlled trials (RCTs) as of April 2012. Thus, CIMT has been tested more than any other type of UE training approach. Since children are not as easily motivated to perform activities of daily living or part practice for sustained periods of time in the way that adults are, the overall approach must be adapted to focus on age-appropriate activities that sustain interest for long periods. The protocol used for adults, with a cast on the less-affected UE and training for three weeks has been used in children (Taub et al., 2004, 2007). It has been suggested that deviations from this protocol result in compromised intensity (Taub et al., 2007). However, effect sizes used for comparison were performed on different measures, none of which were validated for use in this population. Without building on each other in a rigorous fashion, modifications have been made that include restraint type, restraint duration, therapy duration, dose frequency, and providers without building on each other. The study designs, age of participants and outcome measures also differ greatly across studies. While overall there is increasing evidence of efficacy irrespective of these differences (Gordon, 2011; Hoare et al., 2007; Huang et al., 2009; Sakzewski et al., 2009), the diversity of protocols makes it nearly impossible to compare across studies to determine the effect of various models. This greatly limits the clinician’s ability to implement a model of CIMT that suits the local environment and meets the needs of children and families. These factors are reviewed below. Knowing the optimal age to conduct CIMT is an important consideration for children, families and providers. The mean age of children in the majority of studies ranges from 2 to 7 years. However, studies have included children as young as 7 months (Taub et al., 2004) to teenagers (Gordon et al., 2006; Sakzewski et al., 2011a). Given the very early age in which brain damage occurs in CP, one would think that there is tremendous potential for recovery (Kennard, 1936) and that ‘earlier treatment is better.’ While there may indeed be certain windows of opportunity that could lead to better outcome, it is now understood that plasticity is more complicated than that. There is only one specific study examining age (Gordon et al., 2006). No difference between outcomes in children aged 4 to 8 years and with those aged 9 to 13 years was found, as seen in Figure 14.2, which shows similar changes in the JTTHF in both age groups. However, it may well be that the older children had greater attention and motivation, and thus worked harder for the same gains. In a modified study of restraint schedules, Eliasson et al. (2005) found that in children aged 1.5 to 4 years, the older children actually made greater gains than the younger ones. Sakzewski et al. (2011a) showed the same relationship between age and outcome in children aged 5 to 16 years. Yet, other studies have shown the opposite (Eliasson et al., 2005; Hoare et al., 2013). Figure 14.2 Mean±SEM time to complete the six timed items (writing excluded) of the Jebsen–Taylor test of hand function for the younger (n=12) and older (n=8) age groups at each testing session. Faster times correspond with better performance. [Modified from Gordon AM, Charles J, Wolf SL. Efficacy of constraint-induced movement therapy on involved-upper extremity use in children with hemiplegic cerebral palsy is not age-dependent. Pediatrics 2006;117:e363–373, with permission from the American Academy of Pediatrics.] Despite the discrepancies, there is neuroanatomical data in the developing infant and kitten to suggest that the best time to start treatments eliciting movements of the more affected UE such as CIMT may well be earlier than studied to date. Unilateral damage to cortical motor areas results in a failure of the affected corticospinal tract (CST) to secure and maintain normal terminations in the spinal cord (Eyre et al., 2007; see Martin et al., 2011). Termination of the CST in the spinal cord requires activity-dependent competition between the two sides of the developing motor system. During normal development, the CST initially projects bilaterally from both motor cortices at birth, and is pruned into the mature contralateral projection pattern seen in adults during the first few years of life (Eyre et al., 2001). Damage to one side of the motor cortex, as occurs often in unilateral CP, results in aberrant organization of the motor system, with the damaged side failing to establish normal CST connections. Concurrently, the undamaged/less-damaged CST maintains excessive bilateral projections that invade the normal termination zone of the contralateral spinal cord. A non-invasive brain stimulation technique, transcranial magnetic stimulation (TMS), has been used to study the integrity of the CST in children with unilateral CP (Eyre et al., 2001, 2007; Staudt et al., 2004) (see Chapters 2 and 3). Initially after the neurological insult, the CST from the damaged hemisphere is readily excitable by TMS, indicating connectivity to the spinal cord. The excitability of the damaged CST decreases in a time-dependent fashion, even years after the initial damage. Concurrently, the hemisphere contralateral to the damage increases in excitability. Specifically, the ipsilateral stimulation response is heightened, suggesting a strengthening of these projections. The strengthening of ipsilateral CST connections is believed to be a consequence of activity-dependent competition between the two hemispheres—the more active side ‘wins out’ over the less active (damaged) side (Eyre et al., 2007; Martin et al., 2011). Consistent with this TMS data, neuroanatomical and behavioural studies in a feline model of unilateral CP indicate that behavioural deficits that emerge with unilateral CP are caused by aberrant organization of the CST that appears to increase with maturation (Martin et al., 2011). Balancing activity of the two hemispheres immediately after unilateral brain trauma by pharmacologically decreasing activity of the uninvolved side, restores motor function, normal anatomical connectivity of the CST and the motor representational map in primary motor cortex (Martin et al., 2011). This model points to the importance of increasing activity of the involved UE, a principle incorporated into both CIMT and bimanual training (see below). It encourages very early training of the affected UE to balance activity between the two sides before the less-affected CST ‘outcompetes’ the affected CST for synaptic space in the spinal cord. The time window for restoring CST connectivity by balancing activity may be rather narrow, occurring at a very young age. However, the converse is also true: restricting movement of the less-affected UE for long periods of time would disrupt the normal development of the CST, potentially resulting in impaired function of the better hand. Thus, restriction of the less-affected UE should be done in moderation at an early age. Modified schedules with just two hours per day of CIMT have been shown to be effective (Eliasson et al., 2005, 2011; Wallen et al., 2011). An important point is that CIMT should not be viewed as a one-time opportunity whereby the less-affected UE is restrained as much as possible (e.g., with a cast) and the highest possible intensity is provided regardless of age. In fact, there is increasing evidence that CIMT can be provided multiple times during development without reducing the magnitude of subsequent change (Fig. 14.3) (Charles and Gordon, 2007; Gordon et al., 2011). Figure 14.3 Mean±SEM time to complete the six timed items (writing excluded) of the Jebsen–Taylor test of hand function immediately before (pre-test 1) and after (post-test 1) the first intervention, and immediately before (pre-test 2) and after (post-test 2) the second intervention one year later. Faster times on the Jebsen–Taylor test correspond to better performance. Note that gains are retained one year after the initial CIMT intervention, and the times improve further after the second intervention. [Modified from Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2007;49:830–838, with permission from John Wiley and Sons.] Restraints used during CIMT include plaster casts worn full time (Case-Smith et al., 2012; Taub et al., 2004, 2011), slings (Charles et al., 2001, 2006; Gordon et al., 2006, 2011), splints (Brandão et al., 2010; Park et al., 2009), and mitt/gloves (Eliasson et al., 2005, 2011; Hoare et al., 2013; Sakzewski et al., 2011a). The cast restricts all movement and does not allow the participant choice to remove it. The other types of restraint allow movement of the less-affected UE (and in the case of a splint or mitt, the restrained UE could be used as an assist). These restraints could be removed by an unco-operative child, although few reports of this occurring exist. In our experience with over 80 children participating in CIMT with a sling, only one (an 8-year-old girl with behavioural problems) refused to wear the restraint. Despite the diversity of treatments and the fact that there are no direct comparison studies, there is no evidence to suggest that one type of restraint is more effective than another in eliciting better affected UE outcomes. Thus, comfort and safety should be a key factor in restraint selection. Despite the large number of CIMT studies, overall, little description is typically provided about the specific movements and activities practiced, largely because of the space constraint of journals. Methodology papers mainly focus on information about RCT designs and procedures (Aarts et al., 2010; Boyd et al., 2010; Facchin et al., 2009) although some treatment details are provided. Early CIMT models (Case-Smith et al., 2012; Taub et al., 2004) involved shaping (called ‘part practice’ in the motor learning literature), which involves approaching a behavioural objective (task) in small steps by successive approximation (Skinner, 1968). The task is made more challenging as the child improves, taking into consideration their abilities. Other models simply involve engaging the children in play and functional activities (Bonnier et al., 2006; Eliasson et al., 2005) without regard to shaping, and in many studies this is not specified. There have not been any studies that examined the roles of specific ingredients such as shaping in children with CP, but nearly all studies suggest efficacy. Since all CIMT approaches involved practice of motor activities, intensity of training may well be more important than ingredients (how practice is provided), at least at the high intensity CIMT is normally provided. The choice of activities to engage children in is important. To engage the child in active intervention and to maintain their attention and effort, we established a battery of motor activities that elicit the general movement behaviours of interest that includes a range of functional and play activities (Gordon et al., 2005). The activities are age-appropriate and can all be performed unimanually. Activities are selected by considering: (1) joint movements with pronounced deficits; (2) joint movements that therapists believe have greatest potential of improving performance; and (3) child preference for activities that have similar potential for improving identified movements. Therapists usually begin with a quick task which results in successful completion to build confidence. The task is made progressively more challenging as the child’s performance improves by requiring greater speed or accuracy, additional movement repetitions or performance-sensitive adaptations. We adapt task constraints to allow success, and remove them as skill improves using specific criteria. Age-specific structured feedback (knowledge of results) is provided to motivate the child. Nevertheless, whether such structured interventions are advantageous over just play and functional practice with less structure is not known. Table 14.1 illustrates the types of activities we have used for children aged 3.5 years and above, with examples of targeted movements and how the constraints are graded to vary the difficulty. There are eight categories, consisting of board games (e.g., Candyland®, Monopoly®), card games (e.g., Old Maid®, Uno®), manipulative games (e.g., Don’t Break the Ice®, Battleship®), puzzles, arts and crafts (e.g., drawing, painting), functional tasks (e.g., eating, dressing), full upper extremity motor activities (e.g., throwing a large ball, Scatch®), and video gaming (Gordon and Okita, 2010). We view the choice of specific activities as less important than the movements they elicit. For example, board games could be used to encourage wrist supination and extension, precision grasp and grasp maintenance. Video gaming consoles (such as the Nintendo Wii®) can be used to induce full UE movements and to challenge postural control (especially if seated on a fitness ball). Table 14.1
Constraint-induced therapy and bimanual training in children with unilateral cerebral palsy
Task-oriented training
Constraint-induced movement therapy
Constraint-induced therapy in children with unilateral CP
Age of CIMT participants
Restraint type
Ingredients of CIMT
Activity category
Targeted movements
Graded constraints
Board games
Supination, wrist extension, precision grasp, maintaining grasp through changes in spatial orientation
Active wrist extension—position deck of cards to elicit wrist extension and grade difficulty by changing position of deck
Card games
Supination, precision grasp
Precision grasp—less difficult when cards are bevelled on deck for easier grasp. Increase difficulty by not bevelling the cards
Functional tasks
Wrist extension, supination and pronation
Supination and pronation—for turning key in lock, vary starting position of key to grade from using only supination to using both supination and pronation
Whole upper extremity
Shoulder flexion, shoulder abduction, shoulder external rotation, wrist extension
Shoulder flexion—elicit shoulder flexion by moving child from easier position stabilized against a wall, to free standing position that requires more control
Manipulative games
Finger individuation, precision grasp, wrist extension, modifying grasp to accommodate various objects
Precision grasp—to increase difficulty provide child with increasingly smaller or more complex objects to manipulate
Puzzles
In-hand manipulation, precision grasp, release accuracy
Release accuracy—once competency in releasing puzzle pieces is attained, increase difficulty by introducing a puzzle with smaller pieces
Arts and crafts
Supination, precision grasp, maintaining grasp through changes in spatial orientation
Maintaining grasp—begin child at an easier level with a built-up brush, and increase difficulty by removing assist. Smaller brushes can be introduced
Video gaming
Wrist supination and extension, shoulder flexion, shoulder abduction dexterity, shoulder external rotation
Wrap controller with tape, sit on fitness ball ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Constraint-induced therapy and bimanual training in children with unilateral cerebral palsy





