What is Osteoporosis?
Introduction
Osteoporosis is a condition characterized by low bone mineral density and compromised microarchitectural integrity leading to structural failure of the skeleton even at low loads. The clinical consequence of osteoporosis is fracture.
Osteoporosis is common in the population and it has been estimated that 1 in 2 women and 1 in 5 men above the age of 50 years will suffer a fracture during their remaining lifetime. These fractures occur usually after the age of 65 years, the mean age of hip fracture in Sweden being 81 years. The number of people over 65 years and over 80 years is increasing dramatically. It is estimated that the population over 65 years of age will increase from the current 16% to between 20 and 25% by 2050 in Europe. There is also a predicted dramatic increase in the older population in less developed countries. Risk factors are also increasing such as reduced physical activity, less balanced diets, smoking, and alcohol consumption. These demographic changes are predicted to result in a dramatic increase in fractures with subsequent morbidity and mortality.
Definition of osteoporosis
Osteoporosis is a condition that is characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk1. Bone quality and its strength is not only a reflection of density and microarchitecture but there are other factors that influence this and fracture risk2 which need to be considered.
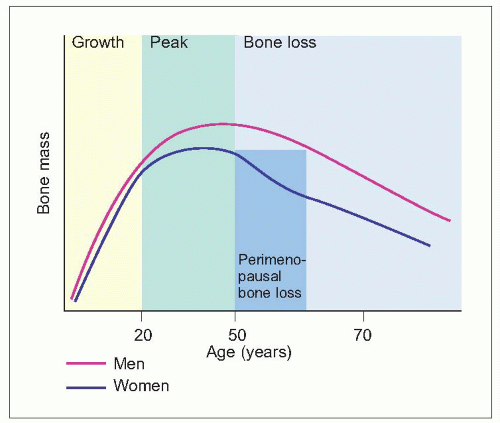
Bones grow in size during the first two decades of life, with an acceleration during adolescence (1.1). This is followed by a period of consolidation. Peak adult bone mass is reached at about the age of 35 years for cortical bone and a little earlier for trabecular bone. Bone mass subsequently declines with ageing. This is a universal phenomenon, occurring in both sexes and in all races. At all ages, women have less bone mass than do men.With ageing this difference becomes more pronounced.
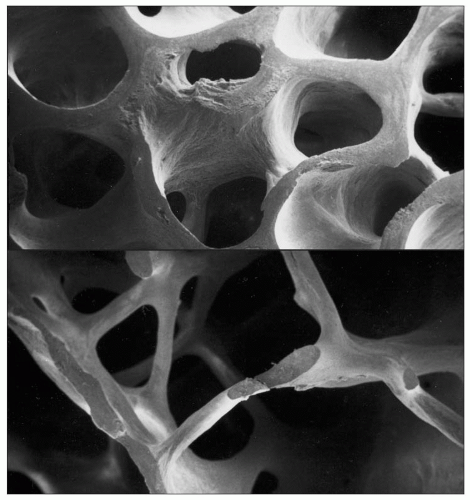
With ageing there are changes in the microarchitecture of bone (1.2, 1.3). There is thinning of the cortex and of trabeculae, and a loss of connectivity, in particular of the horizontal trabeculae. The operational definition of osteoporosis is in terms of bone mass1 (Table 1.1), although this is not the only factor that determines bone strength or fracture risk. Bone mass or density is measured by dual energy X-ray absorptiometry in the hip or spine, but various techniques can be used at different sites which correlate with this.
Aetiology of fractures
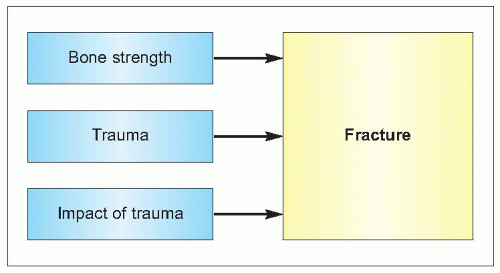
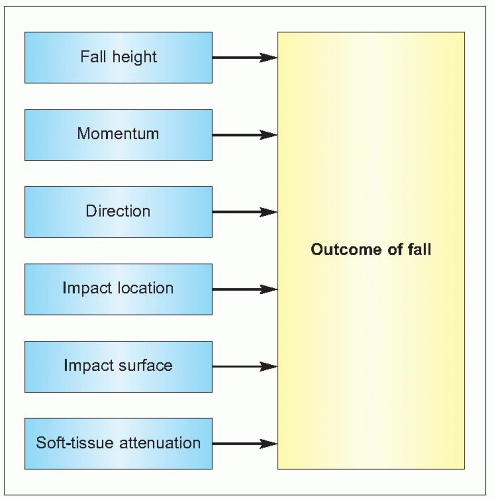
The cause of fractures is multifactorial. The principal factors that interact are the strength of bone, the event of an injury (usually a fall), and the force on the skeleton from that injury (1.4). Various factors influence each of these.
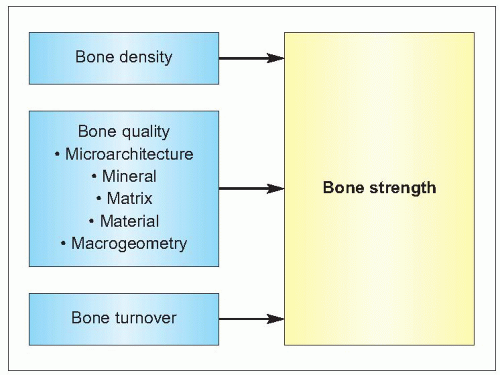
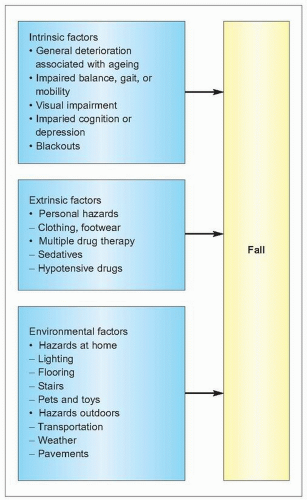
Bone strength is dependent on the structural and dynamic characteristics of the bone – its density, quality and rate of turnover (1.5). The quality of bone tissue relates to its composition and microstructure, whereas its quality as an organ depends also on its macrostructure. Fractures usually follow an injury, in particular peripheral fractures. In young people this is usually related to sport or road traffic accidents, but in an older person typically is as a result of a fall. Falls become increasingly common with age and the causes are many (1.6). The causes can be considered as intrinsic, extrinsic, and environmental factors but usually there are several factors that contribute to a person falling. As people age, the impact of any fall increases for a variety of reasons (1.7), which increases the chance of a consequent fracture. These factors will be considered in more detail.
Factors that influence bone strength
Bone is an organ that gives form to the body, supporting its weight, protecting vital organs, and facilitating locomotion by providing attachments for muscles to act as levers (1.8). It also acts as a reserve for ions, especially calcium and phosphate, the homeostasis of which is essential to life. It is composed of cells and extracellular matrix, like other connective tissues, but the matrix has the unique ability to be calcified.
The strength of a bone and its ability to perform these physical functions depend on its structure and the intrinsic properties of the materials of which it is composed. The amount of bone (bone size, mass, and density), its spatial arrangement (shape, geometry, and microarchitecture), its composition (intrinsic properties of bone materials), and its turnover (rate and balance of formation and resorption) are all such determinants of its ability to perform mechanical functions and to resist fracture.
Table 1.1 WHO definition of osteoporosis | |
|---|---|
|
Bone structure
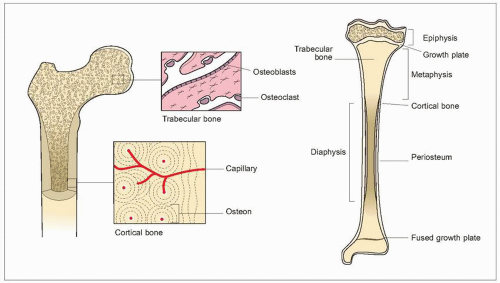
Bones can be conveniently divided into flat bones such as the scapula, skull, and pelvis, and tubular bones which include the limb bones and vertebral bodies. The dense outer surface or cortex is composed of compact bone and the centre or medulla is braced by narrow plates or trabeculae, a construction which gives maximum strength for minimum weight (1.8). In the interstices of the medulla lies the bone marrow, where bone cells are in close contact with haemopoietic cells.
Cortical bone
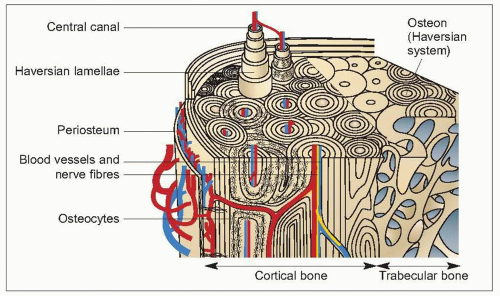
Cortical (compact) bone (1.9) constitutes 75-80% of the skeletal mass. It forms the outer surface of all bone but the majority is found in the shafts of tubular bone. Compact bone is composed of lamellae which are concentrically arranged around a small central canal to form a Haversian system or osteon. Between the lamellae are osteocytes lying in lacunae which are connected with each other and with the central canal by fine canaliculi. Osteocytes lie no more than 300 μm from a blood vessel; the average cross-sectional diameter of a Haversian system is 500 μm. The Haversian systems, which may be up to 5 mm long, run parallel to the long axis of the bone, branching and communicating with each other. There are also interstitial lamellae between the Haversian systems, and circumferential lamellae which encircle the inner and outer surface of the bone.
Periosteal vessels penetrate compact bone through nutrient canals to supply the marrow, and branches of these form the intracortical vessels which lie, along with the venules, within the Haversian canals. The interconnecting canaliculi between the osteocytes allow for rapid movement of fluid for their nutrition and humoral intercommunication.
Haversian systems are formed either by the deposition of new bone on the endosteal or periosteal surfaces of cortical bone (primary osteons) or by osteoclasts cutting tunnels (cutting cones) into bone with subsequent deposition of new bone by osteoblasts (secondary osteons). The latter process is found in bone that is remodelling itself, and the outer limit of a secondary osteon can be identified by a cement line which separates it from adjacent bone.
Trabecular bone
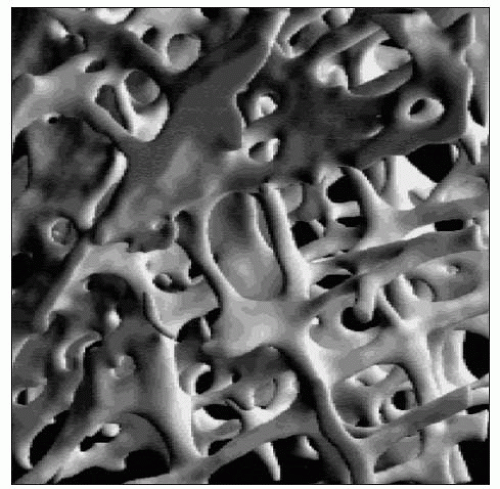
Trabecular bone (1.10) is a rigid meshwork of mineralized bone which forms the greater part of each vertebral body and the epiphyses of the long bones, and is present at other sites such as the iliac crest. It contributes 20% of the total skeletal mass, but 65-70% of the total bone surface. Complete struts are called trabeculae, but incomplete spicules are also seen. The trabeculae usually lie so as to resist deformational stresses (either from weight bearing or from muscle activity) and their number, size, and distribution are related to these forces. The vertical trabeculae are usually thicker but strength is given by the cross-bracing horizontal trabeculae. Trabecular bone provides a large surface area and is the most metabolically active part of the skeleton, with a high rate of turnover and a blood supply that is much greater than that of compact bone. It acts as a calcium reservoir.
Trabeculae have a lamella arrangement but less often contain osteocytes. Growth occurs on their surfaces which are covered by a layer of osteoid (that is, unmineralized matrix), which is produced and subsequently mineralized by surface osteoblasts. Occasional osteoclasts lie on their surfaces in shallow pits known as Howships’s lacunae.
Distribution of different types of bone
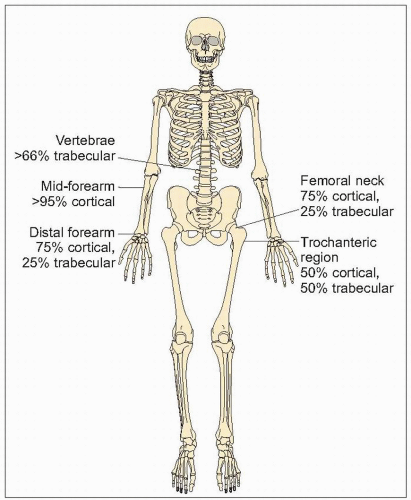
The proportions of cortical and trabecular bone differ at different sites in the skeleton (1.11). Trabecular bone is predominant in the vertebrae and femoral head, but cortical bone predominates at the distal radius and femoral neck. The intertrochanteric area of the femur is 50% cortical and 50% trabecular bone. This distribution and differential loss of cortical and trabecular bone in different scenarios accounts in part for the occurrence of different fractures in different situations: cortical bone loss predisposes to peripheral fractures such as of the hip and wrist, whereas trabecular loss predisposes to vertebral fractures.
Bone composition
The fundamental constituents of bone are the cells and the extracellular matix.
Bone cells
Osteoblasts
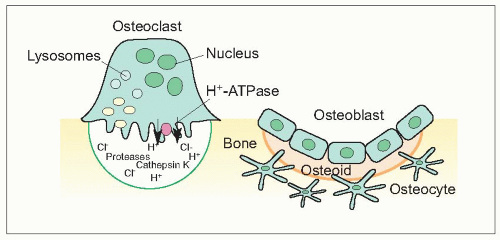
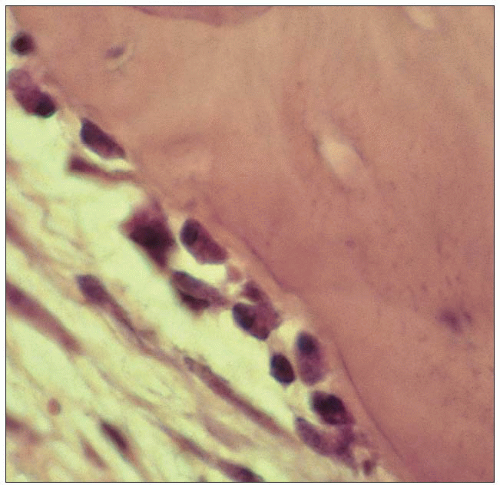
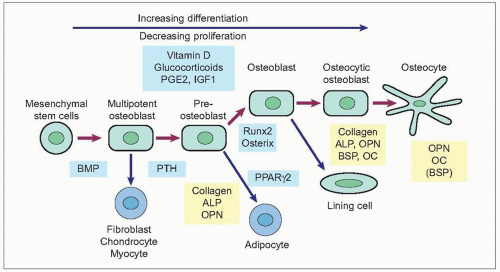
Osteoblasts are responsible for producing bone matrix constituents, chiefly collagen and noncollagenous matrix proteins that form osteoid (1.12, 1.13). They control mineralization of bone. They originate from bone marrow stromal or connective tissue mesenchymal stem cells which proliferate and differentiate into preosteoblasts and then mature osteoblasts, after being subject to different stimulations of local growth factors and transcription factors (1.14). Osteoblasts are found in clusters of up to about 400 cells at a bone-forming site. Surface osteoblast or lining cells line inactive trabecular surfaces. Activated osteoblasts line the layer of bone matrix they are making, the osteoid surface, prior to calcification. Their cellular structure reflects their high synthetic and secretory activity with a well-developed rough endoplasmic reticulum and large Golgi complex and a number of more or less bone-specific proteins, collagen type I in particular, are secreted. The plasma membrane of the osteoblast is rich in alkaline phosphatase (ALP) and the ALP activity increases early in the mineralization phase. Osteoclasts have cell surface receptors for hormones including parathyroid hormone, vitamin D, and oestrogen, but also cytokine receptors. There is a close linkage between osteoblast and osteoclast activation, and cells of osteoblast lineage secrete cytokines that participate in osteoclastogensesis. Osteoblasts express cytokines on their surface including RANK ligand (RANKL) which, through interaction with RANK, promotes bone resorption. Osteoprotegerin is also secreted by osteoblasts, which is a decoy RANK receptor that can inhibit osteoclast formation.
After forming bone, some osteoblasts are embedded in the mineralized matrix and become osteocytes, some remain on the surface and become bone-lining cells, whereas others will undergo apoptosis (programmed cell death).
Osteocytes
Osteocytes are embedded deep within bone in small lacunae, having originated as osteoblasts and becoming trapped in the matrix they produced. They have numerous long cell processes which are in contact with other osteocytes and lining cells on the bone surface. They are surrounded by the periosteocytic space which is filled with extracellular fluid. Osteocytes have a role in maintaining extracellular calcium concentration. They may also act as mechanoreceptors and in the local activation of bone remodelling.
Osteoclasts
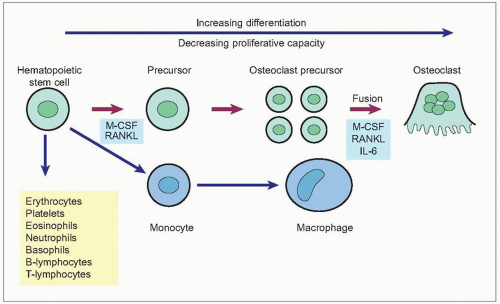
Osteoclasts are responsible for bone resorption. They are giant, multinucleated cells usually found in contact with calcified bone surface within lacunae that result from their resorptive activity (1.15). An activated resorption site may contain from one to five osteoclasts. Osteoclasts have a different origin from osteoblasts. They are derived from hematopoetic stem cells and are related to macrophages (1.16). Mature osteoclasts are formed by the fusion of osteoclast precursors. Osteoclast differentiation is promoted by the interaction of RANK expressed on osteoclasts and RANKL. They have abundant Golgi complexes, mitochondria, and transport vesicles containing lysosomal enzymes. Osteoclasts form sealed bone-resorbing compartments next to the bone surface, with a ruffled border formed by deep foldings of the plasma membrane facing the bone matrix. They undergo apoptosis after they have finished resorbing bone.
Table 1.2 Composition of bone | |||
|---|---|---|---|
|
Bone matrix and mineral
The extracellular matix is a ‘composite’ in materials science terms, a matrix comprised of collagen and ground substance that is mineralized. Crystals of hydroxyapatite are precipitated on the collagen fibres. The mineral phase gives compressive strength and rigidity, but it is the fibrous organic matrix that gives bone its resistance to tractional and torsional forces. The mineral phase accounts for up to 70% of adult bone.
Collagen forms 90% of bone matrix, of which type 1 is the major component. Noncollagenous proteins form the ground substance, primarily glycoproteins and proteoglycans, but there are other matrix proteins present in small amounts that have important although not fully characterized roles (Table 1.2). Most but not all of these noncollagenous proteins are synthesised by bone cells.
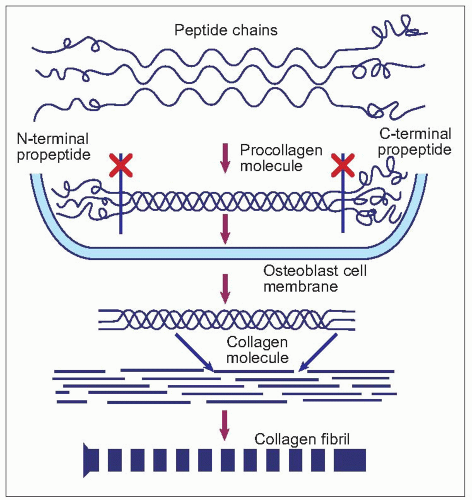
Type I collagen is formed in bone from the combination of two α-1 and one α-2 collagen polypeptides containing hydroxylated proline and lysine residues (1.17). It is secreted as procollagen from the osteoblast, when the amino-terminal and carboxy-terminal regions are cleaved. Type I collagen is helical; the nonhelical domains at the amino- and carboxytermini are known as the N-telopeptide and C-telopeptide regions. The structure of type I collagen is stabilized by side chains of hydroxylysine residues which condense to form pyridinium rings so that pyridinium cross-links are formed, connecting three different collagen molecules. These crosslinks are described as pyridinoline or deoxypyridinoline cross-links, depending on the combination of hydroxylysine and lysine side residues3,4. The cross-linking is specific for each of the N- and C-terminal telopeptide regions, and is also relatively bone-specific5. The orientation of the collagen fibres alternates from layer to layer in adult bone, which gives the typical lamellar structure seen by polarizing light or electron microscopy.
Bone growth
Tubular bones grow in length by ossification at the metaphysis at cartilage growth plates (endochondral ossification). The cortex grows in diameter by subperiosteal deposition accompanied by endosteal resorption. This process leads to enlargement of the marrow cavity. Flat bones develop by intramembranous bone formation. An ossifying growth plate can be divided into functional zones. Chondrocytes initially proliferate and then actively synthesize matrix, the cells having an internal arrangement typical of secretory cells. Next, the cells hypertrophy, compressing the surrounding matrix. In the next zone calcification is found, initially with small isolated clusters of crystals which coalesce to an almost solid mass at the cartilage-bone junction. Capillary buds, osteoprogenitor cells, and osteoclasts then penetrate and resorb this somewhat amorphous mineralized matrix and is replaced by new bone formed by the osteoblasts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree