CHAPTER 10 Viscerosensory pathways in the brain
Introduction
In addition to providing the critical information necessary for homeostatic functions, viscerosensory signals can potently affect cognitive and emotional functioning. For instance, we remember emotionally arousing events better than things that are routine, and this is dependent upon vagal sensory neurons detecting the levels of stress hormones (epinephrine) circulating in the body (Miyashita & Williams 2004). Vagal sensory neurons in turn drive brain pathways related to stress and arousal that enhance memory formation. In addition, infections in the body can cause symptoms of depression and anxiety (Dantzer 2004, Lyte et al. 2006). Whereas somatosensory information from joints, skin, muscles provide us with a sense of where we are in space, viscerosensory information contributes not only to emotional states (Wiens 2005), including fear, anxiety, excitement etc. but also may contribute to the perception of our core “selves” (Damasio 2003). The demands of regulating our bodily functions are intimately tied into our ability to cope with both physiological and psychological challenges. Successful resolution of such challenges is critical to survival, thus homeostatic systems must be able to influence psychological aspects of motivation. In this way, by providing information about the bodily states, viscerosensory systems constitute a principal conduit for mind and body interactions. However, perturbations in balance/interactions of homeostatic and psychological states contribute to functional disorders, such as irritable bowel syndrome and chronic pain syndromes (Bonaz 2003, Mayer, Naliboff & Craig 2006). Targeting therapeutic interventions toward restoring optimal functioning of viscerosensory systems could improve outcomes of these disorders.
It is becoming increasingly clear that manual therapies (MT) exert actions beyond the specific joints and spinal segments stimulated (Schmid et al. 2008), likely involving supraspinal (brain) and neuroendocrine components. Indeed, MT can influence autonomic functions (heart rate and respiration) and induce analgesia at body sites away from site of MT (reviewed in Schmid et al. 2008), and these actions likely contribute significantly to MT efficacy. However, the specific mechanisms and pathways that mediate the effects of MT on autonomic and pain-related functions are not well-established. As Bialosky et al. (2008) point out, understanding mechanisms of MT is critical to targeting of treatment, predicting outcome, and increasing acceptance by health care providers. Thus, delineating brain mechanisms influenced by MT will provide important insights and enhance efficacy of these modalities.
Organization of ascending viscerosensory pathways
Origins of viscerosensory pathways
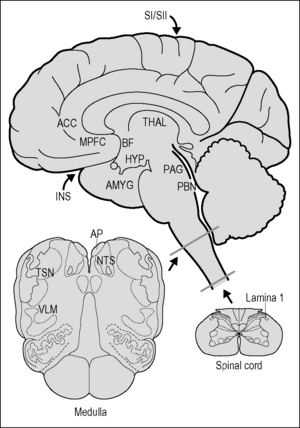
Parallel pathways originating in the spinal cord and brain stem collect information from all of the tissues of the body and propagate the signals to brain regions involved in homeostasis. Traditionally, viscerosensory pathways have been conceptually limited to those derived from the nerves innervating thoracic, abdominal, or pelvic organs and tissues. However, it has recently been pointed out by Craig (2002, 2003) that nerve fibers innervating skin and blood vessels, etc. are functionally and anatomically more similar to viscerosensory nerve fibers (also called ‘interoceptive’) than somatosensory fibers such as touch, which provide ‘exteroceptive’ information. For instance, all of these fibers, regardless of their target of innervation, are thinly myelinated or unmyelinated, and most if not all respond to inflammatory signals, including prostaglandins, bradykinins, and cytokines. Thus, signals from all bodily tissues contribute to the representation in the brain of ‘condition of the body’ (e.g., health, damaged tissues, etc.), and converge at multiple levels of the neuraxis (Cameron 2001, Craig 2003). In this chapter, the terms viscerosensory or interoceptive will refer to pathways derived from neurons in spinal lamina 1, the spinal trigeminal nucleus, or the dorsal vagal complex and ventrolateral medulla (Fig. 10.1).
Lamina 1 of the spinal cord
Lamina 1 of the spinal cord collects information from small-diameter primary sensory neurons that signal tissue damage and/or inflammation, temperature, and itch, in all tissues of the body except the head, where analogous fibers run with the trigeminal cranial nerve and terminate in the spinal trigeminal nucleus (Sessle 2005). These fibers project to the autonomic nuclei of the spinal cord and ascend to terminate in the thalamus, while giving off collaterals to the several nuclei in the brain stem, including the nucleus of the solitary tract, the ventrolateral medulla, and the parabrachial nucleus (reviewed in Craig 2003).
Trigeminal sensory nuclei
The trigeminal sensory nuclei monitor pain and immune/inflammatory signals from tissues of the head: tooth pulp, mucosal surfaces of the mouth (including the tongue) and nose, salivary glands, mouth, and skin of the head. The nucleus caudalis of the spinal trigeminal nucleus has many features in common with spinal lamina 1, and serves as the principal – but not the only – site for pain processing (Sessle 2005). Pain-related and viscero-sensory signals from the trigeminal nuclei are propagated via ascending neural projections in the trigeminothalamic tracts to brainstem regions, including the NTS (Takemura et al. 2006) and lateral parabrachial nucleus, on their way to the thalamus (Sessle 2005).
The nucleus of the solitary tract (NTS)
The NTS is best known for its role as the primary sensory relay nucleus for the vagus, glossopharyngeal, and facial cranial nerves, which carry taste and general viscerosensory information from the throat and most of the thoracic and abdominal structures, as well as some pelvic organs. With the area postrema (below), the NTS forms the sensory dorsal vagal complex (DVC). In addition, the NTS receives ascending input from spinal lamina 1 (‘bottom up’) and from the trigeminal sensory nuclei (Takemura et al. 2006), as well as descending input from multiple forebrain regions, including the cortex, paraventricular hypothalamus, and central extended amygdala (‘top down’). Information available to the NTS is not limited to neural input, as it contains receptors for glucocorticoids (Roozendaal et al. 1999) and, via the area postrema and the vagus nerve, senses circulating catecholamines and other hormones that signal psychological stress (Roozendaal et al. 1999) or visceral challenge. Viscerosensory information reaching the NTS is processed locally and appropriate reflexes are generated, and information is relayed to more rostral regions of the brain. The NTS contributes to the mediation of sickness symptoms, notably those associated with sickness behavior, including fatigue (Marvel et al. 2004), modulates cardiovascular and gastrointestinal functions, and plays a critical role in the pathway by which peripheral arousal facilitates memory (Williams & McGaugh 1993). Thus, the NTS operates as a clearing house for a wide variety of viscerosensory signals.
The area postrema
The area postrema is one of the circumventricular organs (CVOs), which are sensory structures located within the brain at various places adjacent to ventricular spaces. In CVOs the blood–brain barrier is weak, allowing cells within them access to substances in the blood that are excluded from the rest of the brain. Such substances include hormones and products of inflammation such as cytokines, which are large lipophobic molecules that do not readily pass the blood–brain barrier, as well as pathogens or pathogen products such as lipopolysaccharide (LPS). CVOs harbor immune cells that produce cytokines during infection or inflammation (Goehler et al. 2006), which are important for the induction of sickness behavior (Dantzer 2004). Unlike the other circumventricular organs, however, the area postrema receives direct viscerosensory input via the vagus nerve, which terminates extensively throughout it (Shapiro & Miselis 1985). This arrangement allows the area postrema access to a uniquely wide variety of peripheral signals: those present in the general circulation, in the cerebrospinal fluid, and, carried by vagal sensory nerves, those arising from distant viscera, e.g., related to local tissue conditions such as inflammation. Area postrema projection neurons propagate these signals to the NTS and to the lateral parabrachial nucleus in the pons (Shapiro & Miselis 1985). Whereas the area postrema is famous as an ‘emetic’ center, it apparently also contributes to EEG synchronization and slow-wave sleep (Bronzino et al. 1976). Thus, signals transduced by the area postrema contribute to ascending viscerosensory pathways and may play a role in brain and behavioral arousal.
The ventrolateral medulla
The ventrolateral medulla (VLM) refers to large neurons that express catecholamines or other substances, including glutamate and neuropeptides, within the reticular formation, an extensive network of large interconnected neurons that tend to form columns rather than well-defined nuclei. The VLM is driven by input from the dorsal vagal complex and spinal lamina 1, and modulates pulmonary and cardiovascular function. Like the NTS, neurons in the VLM projecting to more rostral brain regions contribute to responses to immune and other visceral challenges (Dayas et al. 2001, Gaykema et al. 2007, Sawchenko et al. 2000). In particular, the catecholaminergic neurons that target the hypothalamus respond to circulating cytokines, and provide the primary drive on hypothalamic neuroendocrine systems under conditions of sickness (Sawchenko et al. 2000) and other viscerosensory challenges, such as hypotension or pain (Pan et al. 1999).
Ascending viscerosensory pathways in the brain
Ascending projections from the VLM/DVC derive from at least two neurochemically distinct groups of neurons. The largest group comprises the noradrenergic and adrenergic neurons that innervate structures distributed more rostrally along the neuraxis, in particular the parabrachial, periaqueductal, and dorsal raphe nuclei, hypothalamus, and basal forebrain, including the amygdala and bed nucleus of the stria terminalis (Gaykema et al. 2007, Hajszan & Zaborszky 2002, Herbert & Saper 1992, Peyron et al. 1996), discussed in the following section. Along with the DVC, the VLM seems to provide most of the noradrenergic and all of the adrenergic innervation of the hypothalamus, including the paraventricular nucleus (PVN; contains corticotrophin-releasing hormone, CRH, neurons driving corticosteroid responses) and tuberomammillary neurons (histaminergic neurons), as well as most of the innervation to the basal forebrain (including cholinergic neurons) (Gaykema et al. 2008, Hajszan & Zaborszky 2002, Peyron et al. 1996). Based on these connections, VLM and DVC neurons serve as links between visceral challenges, arousal, and thus potentially affective states. Many of these adrenergic and noradrenergic neurons located in the VLM and DVC become strongly activated by systemic challenge with immune stimulants (Dayas et al. 2001, Gaykema et al. 2007, Sawchenko et al. 2000) and other potentially dangerous viscerosensory challenges, including pain (Pan et al. 1999). Thus, they can be thought of as constituting a specific ‘danger pathway’.
The other group of ascending projection neurons resides in the DVC and expresses a variety of peptides (e.g., glucagon-like peptide-1, cholecystokinin, galanin; Herbert & Saper 1990, Rinaman 2004). These nerve fibers project primarily to the parabrachial nucleus and hypothalamic structures. Although there is less information avail- able on the functional aspects of the non-catecholamine projections than on the catecholamine neurons, they probably contribute to propagation of viscerosensory challenges (because non-catecholamine cells respond to challenges) as well as to other types of homeostatic stimuli.
Targets of viscerosensory projections
Principal viscerosensory targets integrate physiological adjustments associated with mood and physiological or behavioral challenges
Brainstem
Periaqueductal gray (PAG)
The PAG forms a cell-rich area surrounding the cerebral aqueduct in the midbrain. A major function of the PAG involves responses to danger or threats, and it is organized topographically in columns of cells according to specific behavioral and cardiovascular responses to such threats (Green & Paterson 2008). In addition, the PAG contributes to descending pain-modulating pathways targeting the spinal dorsal horn. In the context of real threat of danger (fear), activation of PAG neurons inhibits pain transmission. Interestingly, in the context of anxiety, however, another PAG-derived pathway acts to enhance pain (Lovick 2008). This mechanism may mediate psychological contributions to anticipatory pain, as well as to enhanced pain perceptions in conditions such as fibromyalgia and irritable bowel syndrome.
Diencephalon
Hypothalamus
The hypothalamus, located in the ventral basal part of the forebrain, serves as a principal integrator for psychological ‘top-down’ and viscerosensory ‘bottom-up’ signals controlling physiological regulation (Herman et al. 2005, Sawchenko et al. 2000). The hypothalamus contains populations of neurons that control neuroendocrine aspects of reproductive behavior, responses to physiological and behavioral challenges, and fluid balance via their influence on the pituitary gland. Hypothalamic control over neuroendocrine stress responses is effected via the release of corticotrophin-releasing hormone (CRH), which induces the pituitary gland to release adrenocorticotropic hormone (ACTH), which in turn induces the adrenal gland to release the stress hormone cortisol. Via this HPA axis the brain controls systemic stress responses. In addition to direct control of the endocrine system, hypothalamic neurons contribute top-down control over both branches of the autonomic nervous system, as well as on brainstem neurocircuitry controlling motor aspects of ingestive behavior. As noted previously, ascending viscero-sensory pathways derived from both the NTS and VLM project heavily to the hypothalamus, providing the critical information necessary for induction of appropriate hypothalamic output.
Thalamus
The thalamus constitutes a major link between viscerosensory pathways originating in the spinal cord and brain stem, and the cortical regions associated with viscerosensory perception and integration of viscerosensory input with mood and cognition. Vicerosensory information targets two regions of the thalamus, the ventrobasal complex, and the midline thalamus (Krout & Loewy 2000). The functional specificity observed between projections to the ventrobasal complex and the midline thalamic nuclei continues in their patterns of projections. The ventrobasal complex projects to the insula, a region of cortex located on the inner side of the temporal lobe. These cortical areas seem to be involved in taste and viscerosensory perception, and thus these pathways can be considered as part of the primary sensory pathways for these modalities. In contrast, the dorsomedial nucleus and midline nuclei project to brain regions such as the medial prefrontal cortex, striatum, nucleus accumbens, hippocampus, and amygdala that are involved in integrative functions (discussed below). Thus the thalamus disseminates viscerosensory information to widespread areas of the cortex, as well as to subcortical areas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree