13 Vertebral Body Replacement Devices
13.1 Introduction
Corpectomy procedures are utilized in the treatment of vertebral compression fractures (VCFs) secondary to an array of pathologies (▶ Table 13.1).1,2,3,4,5 Bone grafts such as the tricortical iliac bone crest and fibular strut (▶ Fig. 13.1) had previously been considered the gold standard treatments to fill the space vacated following corpectomy.6,7,8 Although bone graft options have demonstrated clinical efficacy, their use has declined due to an association with postoperative complications such as donor-site pain, immunological rejection, and risk of pseudarthrosis.9,10,11,12,13 Several vertebral body replacement (VBR) devices have been developed with the purpose to reduce the morbidities associated with bone graft implants.9,10,11,14,15
The surgical approach for minimally invasive lumbar corpectomy procedures is similar to that of the lateral lumbar interbody fusion. To mitigate the risk of postoperative nerve root dysfunction, care should be taken to create surgical access anterior to the psoas. Procedures involving the upper lumbar levels provide greater surgical access due to decreased prominence of psoas at these levels. In comparison, procedures involving the lower lumbar levels afford greater difficulty in positioning the retractor anterior to the psoas. As such, more care should be taken when operating at lower lumbar levels to minimize the risk of postoperative nerve root dysfunction.16
13.1.1 Vertebral Body Replacement Device Classification
Selection of implant depends on several considerations such as bone quality, location in the spine, and the number of operative levels.15 To optimize the interface between the vertebral end plates and the VBR device, the implant must feature an adequately sized footprint.17 Additionally, an effective VBR device should restore physiologic load bearing to the anterior column, and restore both vertebral height and lordosis.18 VBR cage implants are becoming the preferred treatment for situations in which the area that needs to be occupied following corpectomy is too large for bone graft.14,15
Table 13.1 Criteria for vertebral body replacement
Indications | Contraindications |
• Spinal tumors • Spinal deformity • Spinal infections • Degenerative lumbar disease • Thoracolumbar burst fractures | • Cervical procedures • Osteoporosis (≥grade 3) • Spondylolisthesis (>grade 2) • Systemic infection |
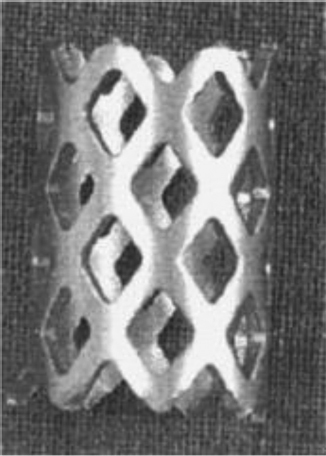
Fig. 13.1 Fibular strut allograft previously used in corpectomy procedures. (Reproduced with permission of © K2 M Fibular Shaft Bone Graft.)
Fig. 13.2 Previously utilized titanium mesh vertebral body replacement (VBR) implant. (This image is provided courtesy of © DePuy Synthes.)
Vertebral Body Replacement Cage Design
The first VBR cages introduced were fixed metal constructs.19 One of the first designs introduced was the mesh cage (▶ Fig. 13.2), which provided an option when use of bone graft was deemed insufficient.8 The hollow structure of the mesh cage provides additional area for cancellous bone graft placement to enhance arthrodesis.8,14,20 Variations in implant length, diameter, and shape (cylindrical, contoured, block) provide improved ability to recreate physiologic vertebral body dimensions and match the sagittal alignment of the prepared vertebral end plates.21 However, mesh VBR implants have also reported instances of failure, regression of lordosis, and subsidence during follow-up period.20
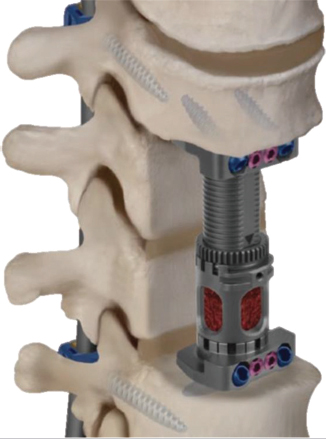
Expandable VBR implants (▶ Fig. 13.3) were developed to improve implant maneuverability and minimize difficulties articulating the implant in the defect space.22 Particularly in multilevel corpectomy procedures, expandable implants afford the option of a minimally invasive approach by allowing for less challenging, nondistracted insertion through a smaller surgical window.13
Vertebral Body Replacement Cage Composition
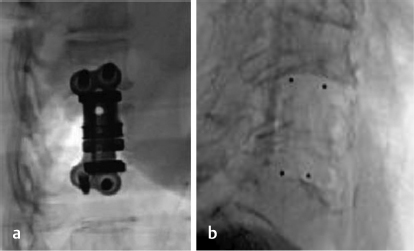
Metal and carbon fiber are the two most common materials utilized in VBR implants. Titanium is the most common metal used, and polyetheretherketone (PEEK) is the most common material in carbon fiber constructs. Both compositions have demonstrated successful outcomes in promoting arthrodesis and restoring vertebral height.23,24,25,26 However, titanium VBR devices have demonstrated increased stability and reduced micromotion compared to PEEK implants.27 In comparison, radiolucent PEEK implants allow for improved analysis of arthrodesis and have proven useful in patients with metal allergies (▶ Fig. 13.4).24,28,29,30,31 PEEK also provides a stiffness and elasticity comparable to normal patient physiology, which have been thought to enhance the rate of arthrodesis.29 Varying evidence exists regarding a superior option with regard to fusion rates between titanium and PEEK implants.25,28,31 As such, further research is necessary to determine the difference in outcomes among material compositions.
Fig. 13.3 Illustration depicting an expandable titanium vertebral body replacement (VBR) implant upon insertion. (Image is provided courtesy of © Globus Medical FORTIFY ICorpectomy Spacer System.)
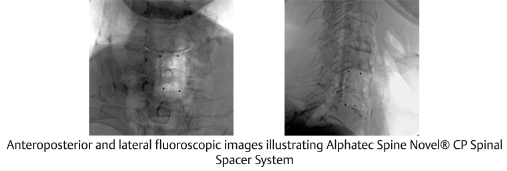
Fig. 13.4 Lateral fluoroscopy illustrating (a) radiopaque titanium and (b) radiolucent PEEK expandable VBR implants. (Images are provided courtesy of © Alphatec Spine Novel CP Spinal Spacer System.)
13.1.2 Efficacy and Outcomes
Through the development of VBRs, corpectomies have been able to provide several advantages over previous surgical treatments for VCFs. Corpectomy procedures have been reported to prevent the loss of long-term vertebral body height correction commonly encountered with cement-only approaches.32,33,34,35 Eck et al assessed complications and long-term outcomes of 66 patients who received nonexpandable VBR implants following corpectomy.23 At 2-year follow-up, the average loss of correction of lordosis was less than 1 degree. The authors also reported no instances of cage failure or extrusion. Ender et al performed an investigation of 15 patients with osteoporotic thoracolumbar fractures who received augmentation with expandable titanium mesh cages.36 At 12 months postoperatively, patients experienced significant improvements in both pain and disability. Furthermore, the degree of vertebral height correction obtained postoperatively was maintained at 12-month follow-up, with no incidences of cage migration or cement-related complications. In another study, Noriega et al utilized an expandable titanium implant in 32 patients with VCFs.26 At 1-year follow-up, patients experienced significant improvements in pain, narcotics consumption, disability, and quality-of-life metrics. Although VBR devices have demonstrated improved outcomes compared to prior utilized treatments, implant subsidence remains a concern.37,38 In a study comparing the efficacy of expandable and nonexpandable implants, Lau et al demonstrated that expandable devices were independently associated with higher rates of subsidence compared to nonexpendable implants.37 Furthermore, the authors reported a greater degree of subsidence associated with expandable implants. In order to effectively determine the ideal implant for corpectomy, additional prospective studies comparing long-term outcomes among VBR devices are required.
13.2 Static PEEK VBR Devices
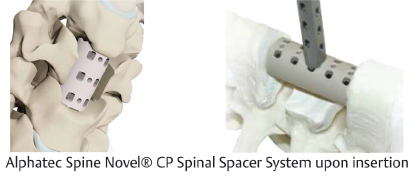
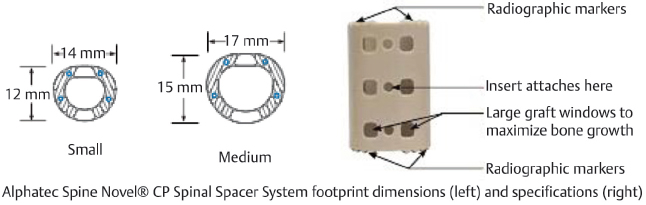
Table 13.2 Alphatec Spine Novel® CP Spinal Spacer System
Design | ||
Type Static | Composition Polyetheretherketone (PEEK) | Design feature Toothed end plates and large bone graft windows improve stability and promote bony fusion |
| ||
Modular aspects and variations | ||
Footprint sizes Small, medium | Lengths available 10–50 mm (1-mm increments) | Lordotic angle 5° |
| ||
Procedures | ||
MIS corpectomy | ||
| ||
Supplemental fixation system | ||
Alphatec Spine Zodiac® Polyaxial Spinal Fixation System | ||
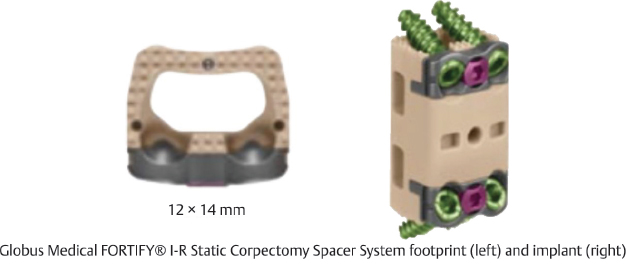
Table 13.3 Globus Medical FORTIFY® I-R Static Corpectomy Spacer System
Design | ||
Type Static | Composition Polyetheretherketone (PEEK) | Design feature Automatic locking system for height stability and preventing collapse |
| ||
Modular aspects and variations | ||
Footprint options 12 × 14 mm | Lengths 15–33 mm (2-mm increments) | Lordotic angle 0°, 3.5°, 7° |
Procedures | ||
MIS corpectomy | ||
Radiographs unavailable | ||
Supplemental fixation system | ||
Globus Medical CREO MIS™ Spinal Fixation System | ||
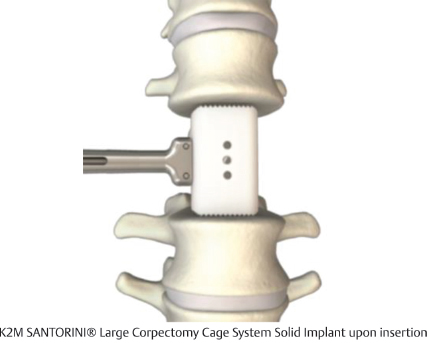
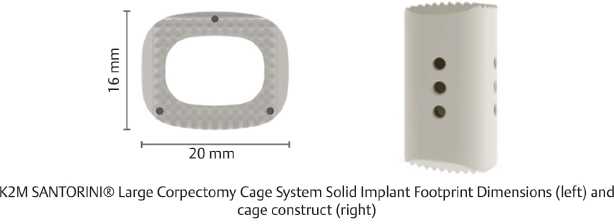
Table 13.4 K2 M SANTORINI® Large Corpectomy Cage System Solid Implant
Design | ||
Type Static | Composition Polyetheretherketone (PEEK) | Design feature Range of heights accommodate smaller anatomy |
| ||
Modular aspects and variations | ||
Footprint sizes 16 × 20 mm | Lengths available 22–34 mm | Lordotic angle 0° |
| ||
Procedures | ||
MIS corpectomy | ||
Radiographs unavailable | ||
Supplemental fixation system | ||
K2 M EVEREST® Posterior Fixation System, K2 M CAYMAN® Lateral Plate Fixation System | ||
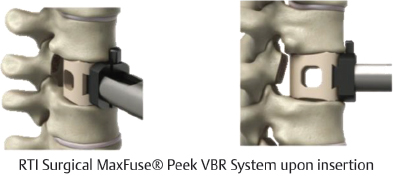
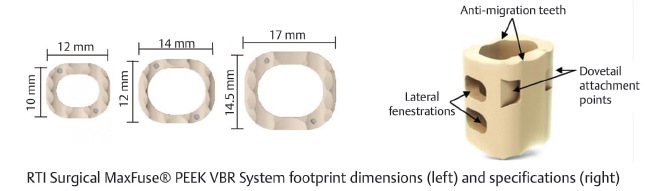
Table 13.5 RTI Surgical MaxFuse® PEEK VBR System
Design | ||
Type Static | Composition PEEK-OPTIMA® from Invibio® Biomaterial Solutions | Design feature Lateral fenestrations and antimigration teeth allow for improved bony fusion and stability |
| ||
Modular aspects and variations | ||
Footprint sizes 10 × 12, 12 × 14, 14.5 × 17 mm | Length 12–46 mm (2-mm increments), 47–65 mm (3-mm increments) | Lordotic angle 8°, 12°, 16° |
| ||
Procedures | ||
MIS corpectomy | ||
Radiographs unavailable | ||
Supplemental fixation system | ||
RTI Surgical’s Streamline® TL or Streamline® MIS Spinal Fixation System | ||
13.3 Static Metal VBR Devices
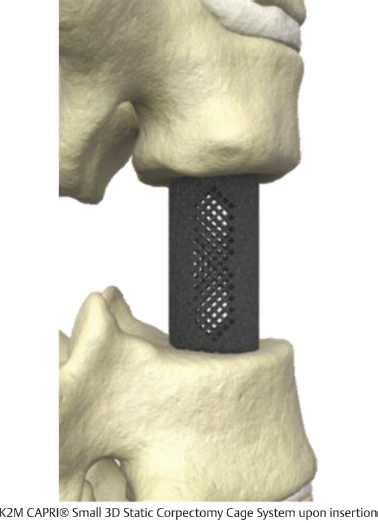
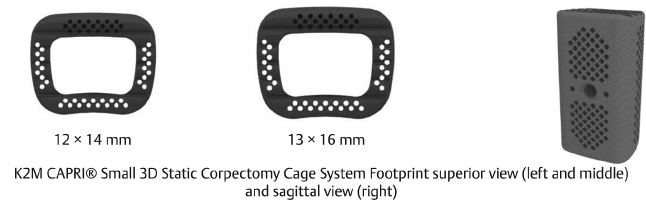
Table 13.6 K2 M CAPRI® Small 3D Static Corpectomy Cage System
Design | ||
Type Static | Composition Titanium | Design feature Pore channels from end plate to end plate enhance bony ingrowth |
| ||
Modular aspects and variations | ||
Footprint sizes 12 × 14, 13 × 16 mm | Lengths available 12–50 mm | Lordotic angle 7° |
| ||
MIS corpectomy | ||
Radiographs unavailable | ||
Supplemental fixation system | ||
K2 M Everest® Posterior Fixation System, K2 M CAYMAN® Lateral Plate Fixation System | ||
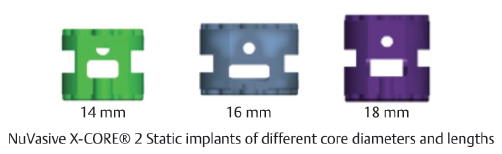
Table 13.7 NuVasive X-CORE® 2 Static VBR
Design | ||
Type Static | Composition Titanium | Implant sizes 16-mm diameter: 12-, 14-mm lengths 18-, 22-mm diameter: 16-, 18-mm lengths |
| ||
Procedures | ||
MIS corpectomy | ||
Radiographs unavailable | ||
Supplemental fixation system | ||
NuVasive XLIF® Decade Plus Lateral Plate System | ||