Venous Thromboembolism Following Total Hip Arthroplasty
Vincent D. Pellegrini Jr
“The possibility of fatal pulmonary embolism after total hip replacement is a hip surgeon’s constant worry … no matter how rare this might be” (1).
—Professor Sir John Charnley, 1979
Introduction
Venous thromboembolism (VTE), specifically pulmonary embolism (PE), is the most common cause of death and one of the most common reasons for readmission unrelated to the surgical site after elective total hip replacement (2,3,4,5,6,7,8,9,10,11). While administrative databases have recently drawn attention to inpatient PE after total hip arthroplasty (THA) and total knee arthroplasty (TKA), nearly 85% of all PE following total hip replacement occur after hospital discharge (5). Collectively, elective THA and TKA are the most commonly performed procedures in the United States, totaling nearly 1 million annually with slightly more than 300,000 cases involving the hip (7,12,13). Moreover, they are increasing in number with the aging of the baby boomer generation and constitute the single largest class of procedures covered by Medicare. Yet, despite advancements in surgical and perioperative care, fatal PE occurs in 0.1% to 0.5% of patients and is responsible for more than 1,000 patient deaths each year following these elective operations (14,15,16). Appropriately, considerable effort has been directed at identifying the optimal method to prevent thromboembolic disease in this patient population. However, use of potent anticoagulants in the perioperative period to mitigate the propensity for activation of thrombogenesis after musculoskeletal operations must necessarily be tempered by consideration of the bleeding risk following major orthopedic procedures where hemostasis is imperfect in the setting of exposed bony surfaces. Orthopedic surgeons typically opt for a perioperative thromboprophylaxis regimen that minimizes bleeding risk, while medical practitioners more often prioritize the absolute reduction of thromboembolic events. Accordingly, recommendations of professional associations have historically disagreed on the matter of VTE prophylaxis and, consequently, the ideal prophylaxis is yet to be determined (17,18). It will undoubtedly represent a risk–benefit balance between the fear of fatal PE and respect for the morbidity of hematoma (19,20,21) and secondary infection resulting from bleeding associated with perioperative anticoagulant use.
Superimposed on this backdrop of controversy around VTE prophylaxis, we now also appreciate that there is a unique propensity for VTE after orthopedic procedures that is largely attributable to activation of the clotting cascade by intravasation of marrow fat into the bloodstream. Instrumentation of the intramedullary canal and pressurization during cementation of the femoral component in THA, combined with intimal injury to the femoral vein, are thought to be responsible for the particularly dramatic predilection for PE that was observed to occur early in the evolution of this operation. As noted by Charnley, the seriousness of this complication is underscored by the possibility of a fatal embolic event following THA despite its successful technical performance. Over the ensuing four decades since the popularization of hip replacement, many technical advances have improved the reliability, safety, and durability of the procedure but the risk of deep vein thrombosis (DVT) and PE persists. In a series of 7,959 THAs performed from 1962 to 1973, Johnson et al. (6) reported an overall PE incidence of 7.89% with fatal PE observed in 1.04% of patients. Since that time, considerable effort has been expended in better understanding the pathophysiology of thromboembolic disease after THA, and even more has been devoted to optimizing its prevention. Accordingly, this chapter will focus on current concepts of VTE, as well as contemporary methods for its prevention.
Epidemiology
DVT is the most common manifestation of thromboembolic disease as a precursor of PE. Historically, in contrast to TKA, the most prevalent form of DVT after total hip replacement was thrombosis in the femoral vein that was segmental in nature and discontinuous with any clot located more distally in the calf. Proximal thrombi, specifically segmental clots in the femoral vein at the level of the lesser trochanter, predominate after THA and are likely initiated
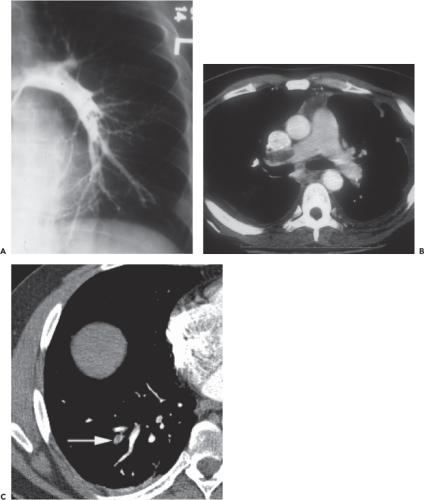
by intimal injury resulting from torsion of the vein during femoral preparation (Fig. 29.1). Such injury has been shown to occur regardless of surgical approach (22). Traditionally, PE was diagnosed by pulmonary arteriography but is now most sensitively identified by multiplanar CT of the chest (Fig. 29.2), which has arguably resulted in overdiagnosis of clinically insignificant subsegmental peripheral thrombi (23). The prevalence of PE is difficult to quantify and its clinical importance is difficult to ascertain, but in the general population the frequency of autopsy-proven fatal PE is 2.5 times that of symptomatic nonfatal PE.
by intimal injury resulting from torsion of the vein during femoral preparation (Fig. 29.1). Such injury has been shown to occur regardless of surgical approach (22). Traditionally, PE was diagnosed by pulmonary arteriography but is now most sensitively identified by multiplanar CT of the chest (Fig. 29.2), which has arguably resulted in overdiagnosis of clinically insignificant subsegmental peripheral thrombi (23). The prevalence of PE is difficult to quantify and its clinical importance is difficult to ascertain, but in the general population the frequency of autopsy-proven fatal PE is 2.5 times that of symptomatic nonfatal PE.
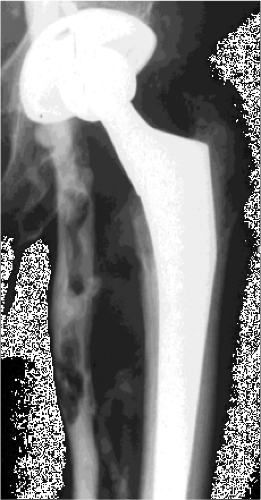 Figure 29.1. Contrast venogram showing segmental proximal femoral vein thrombosis at the level of the lesser trochanter after THA. |
Moreover, DVT may be solely responsible for other morbidity related to chronic venous insufficiency. Five years after surgery, signs and symptoms of postthrombotic syndrome were found in 67% of patients with asymptomatic, venographically confirmed postoperative DVT, compared with 32% of patients who had a negative postoperative venogram (24). Yet, reports of clinically important postthrombotic syndrome are rare among orthopedic centers performing large numbers of hip replacements. Postthrombotic morbidity is more common after idiopathic spontaneous DVT than after provoked postoperative DVT (24) probably because thrombosis in the latter circumstance is more commonly nonocclusive and flow past the thrombus is maintained.
Historically, the risk of DVT in an unprotected patient was 70% to 84% after THA or TKA; the risk of symptomatic PE approached 15%, and the risk of fatal PE was 1% to 3.4% (3,25). Coventry et al. (3) reported a 3.4% fatal PE rate after 2,012 consecutive THAs from 1969 to 1971; the average duration of surgery was 2.4 hours and the average blood loss was 1,650 cc. These patients were on bed rest for 1 week and the mean postoperative hospital stay was 3 weeks. Since that time, the incidence of DVT after total joint arthroplasty has been substantially reduced. The observed decrease is widely accepted as being attributable to the implementation of several pharmacologic and mechanical prophylactic regimens as well as improved techniques of anesthetic management and technical advances in the operation itself (26,27). Data from two studies with a 3- to 6-month follow-up revealed a fatal PE rate as low as 0.12% to 0.35% in 4,594 patients who underwent THA without any type of chemoprophylaxis (28,29). Compared with patients in earlier studies, these patients benefited from more rapid postoperative mobilization and a shorter length of hospital stay. While the reduction in PE events is welcome news for patients, the rarity of fatal PE increases the difficulty implicit in demonstrating a statistically convincing reduction in the event rate with any one regimen as compared with another. With further decreases in the likelihood of fatal PE, it will be necessary to balance the incremental benefit of eliminating PE by using potent anticoagulants against the risk of bleeding complications induced by these anticoagulants (29). For example, in 1977 Johnson et al. (6) reported a 1.68% overall perioperative mortality after 7,959 THAs, in the absence of VTE prophylaxis. Two later studies involving a total of 12,769 patients undergoing THA found a similar 1.71% overall mortality rate without VTE prophylaxis (27,28). While prophylaxis for VTE has been the standard of care since a 1986 NIH consensus conference on the subject (30), not surprisingly current methods of mitigation of VTE risk are as variable as the underlying perceptions of its prevalence.
Routine prophylaxis for VTE after THA or TKA became the standard of care in North America after it was recommended by the National Institutes of Health (NIH) Consensus Conference in 1986 (30). Low-intensity warfarin has been the agent most commonly used by orthopedic surgeons during the subsequent three decades (31,32,33). Although warfarin use has considerably reduced the incidence of DVT, screening venography reveals asymptomatic venous system thrombi in 15% to 25% of patients after THA and in 35% to 50% after TKA. A similarly smaller reduction in venographic DVT after TKA than after THA has been observed with the use of newer anticoagulant agents (26). The overall risk of DVT is two to three times greater after TKA than after THA with the use of current prophylactic agents (26). The frequency of clinically significant bleeding events in patients treated with warfarin was found to be reduced with acceptance of low-intensity anticoagulation; the frequency was 8% to 12% when a prothrombin time index of 2.0 was routinely targeted, but it was 1% to 2% when the targeted prothrombin time index was 1.3 to 1.5 (International normalized ratio [INR] 2.0 to 2.5) (34). Although these data suggest that VTE is more refractory to standard prophylaxis after TKA than after THA, 85% to 90% of thrombi after TKA occur below the venous trifurcation in the deep calf veins, and the immediate risk of embolization is much smaller (25). In contrast, the distribution of DVT after THA historically was 40% proximal and 60% distal (3,26). It has been reported for
newer prophylactic agents that fewer than 10% thrombi after THA are located proximal to the trifurcation, with the remainder occurring in the calf (35). Because 85% to 90% of all DVTs after lower extremity total joint replacement occur in the calf when current prophylaxis is used, greater attention is being given to distal thrombotic disease. Longitudinal surveillance studies found that 17% to 23% of these distal thrombi extend to the more proximal veins of the thigh, where they acquire considerable embolic potential (36,37). The relationship between asymptomatic nonocclusive calf clots and late postthrombotic syndrome appearing as chronic venous insufficiency is unclear, but the considerable embolic potential of postoperative calf thrombi suggests that anticoagulant therapy with extended
prophylaxis should be continued for several weeks after surgery (35). DVT in the calf was reported to have a proximal propagation rate of almost 25% in general surgical patients, and symptomatic PE was reported in as many as 31% of patients with untreated deep calf thrombosis after total hip replacement (36,37). Conversely, spontaneous calf DVT in a patient who is ambulatory and has not recently undergone a surgical procedure is unlikely to be a precursor of pulmonary embolism.
newer prophylactic agents that fewer than 10% thrombi after THA are located proximal to the trifurcation, with the remainder occurring in the calf (35). Because 85% to 90% of all DVTs after lower extremity total joint replacement occur in the calf when current prophylaxis is used, greater attention is being given to distal thrombotic disease. Longitudinal surveillance studies found that 17% to 23% of these distal thrombi extend to the more proximal veins of the thigh, where they acquire considerable embolic potential (36,37). The relationship between asymptomatic nonocclusive calf clots and late postthrombotic syndrome appearing as chronic venous insufficiency is unclear, but the considerable embolic potential of postoperative calf thrombi suggests that anticoagulant therapy with extended
prophylaxis should be continued for several weeks after surgery (35). DVT in the calf was reported to have a proximal propagation rate of almost 25% in general surgical patients, and symptomatic PE was reported in as many as 31% of patients with untreated deep calf thrombosis after total hip replacement (36,37). Conversely, spontaneous calf DVT in a patient who is ambulatory and has not recently undergone a surgical procedure is unlikely to be a precursor of pulmonary embolism.
Knowledge concerning the mechanisms of venous thrombosis and patient predisposition to this condition has paralleled our increased understanding of the basic science of coagulation. Fueled by the same information, the relative risks and benefits of prophylaxis, the choice and duration of specific agents in the face of abbreviated hospitalization, the role of routine diagnostic surveillance, and guidelines for treatment of established VTE have come under increasingly intense scrutiny.
Pathophysiology
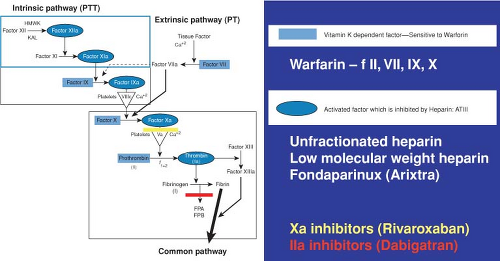
Virchow’s triad, consisting of stasis, hypercoagulability, and damage to the intimal wall, remains the basis of our conceptual understanding of the mechanism of coagulation. Even the slightest of perturbations in the elements of this triad are responsible for abnormalities of thrombosis after musculoskeletal injury, disease, or surgery. Recent discoveries and improved understanding of the clotting cascade form the basis of new therapeutic approaches to preventing and treating thrombotic disease (Fig. 29.3).
Thrombogenesis after Musculoskeletal Injury
It has long been recognized that VTE is more refractory to standard prophylaxis after orthopedic procedures than after general surgical procedures. This phenomenon was first recognized as the inefficacy of subcutaneous heparin for DVT prevention after THA, despite successful VTE prophylaxis with subcutaneous heparin after major abdominal or chest surgery. The discrepancy was found to be secondary to a decline in circulating levels of ATIII (a binding intermediary necessary for the heparins to be clinically effective), that occurred in conjunction with a decline in several other acute-phase reactants after skeletal injury or violation of the medullary canal (as occurs during THA) (38,39).
The findings of Geerts et al. (40) in a study of patients with multiple injuries and an injury severity score higher than 9 underscores the influence of skeletal trauma on the clotting cascade. Contrast venography of 349 patients showed an overall DVT prevalence of 58% and a proximal DVT rate of 18%. Like patients who undergo total joint arthroplasty, most of these patients were asymptomatic; only 3 of 201 DVTs (1.5%) were clinically evident. The overall incidence of DVT associated with specific single-system trauma was 41% for injury to the face, chest, or abdomen; 39% for closed head injury; 66% for lower extremity fracture; and 68% for spinal cord injury. The overwhelming influence of fracture on the clotting cascade was shown by a DVT incidence of 61% with pelvic fracture, 77% with tibial shaft fracture, and 80% with femoral shaft fracture; isolated femoral or tibial shaft fracture was associated with a relative DVT risk almost five times as high as that of the overall
group. Spinal cord injury was associated with an 81% incidence of DVT and an odds ratio of 8.5, compared with the total group (40).
group. Spinal cord injury was associated with an 81% incidence of DVT and an odds ratio of 8.5, compared with the total group (40).
The same investigators subsequently studied the effect of VTE prophylaxis on 344 patients with polytrauma who were randomly assigned to one of two anticoagulant protocols (40). Patients receiving unfractionated heparin had an overall DVT incidence of 44% (60 of 136 patients), compared with 31% of those receiving enoxaparin (40 of 129 patients, p = 0.014); the rates for proximal DVTs were 15% (20 of 136) and 6% (8 of 129), respectively, p = 0.012). These findings highlight the relative ineffectiveness of unfractionated heparins for clinical thromboprophylaxis when circulating levels of ATIII have been reduced. Major bleeding complications were five times more common with enoxaparin therapy (2.9%) than with heparin (0.6%, p = 0.12). Because of their propensity for causing increased bleeding, fractionated heparin should be used cautiously for VTE prophylaxis in patients with polytrauma, and especially in those with closed head trauma, a visceral injury, or an expectation of delayed surgical fracture repair (especially if the pelvis is involved).
Thrombogenesis During Anesthesia and Total Joint Arthroplasty
It is now accepted that the principal thrombogenic stimulus associated with THA occurs intraoperatively. More specifically, femoral preparation is closely linked with intense activation of the clotting cascade as well as torsion or complete obstruction of the femoral vein. Sharrock et al. (41) measured markers of thrombin generation and fibrin formation in circulating blood during THA and found that the process of thrombosis does not begin immediately but is delayed until preparation of the femoral canal. Elevation of prothrombin F1.2, thrombin–antithrombin complexes, fibrinopeptide A, and D-dimer was most pronounced during insertion of the cemented femoral component and continued to increase throughout the first hour after surgery. Mean values for three of the four markers were significantly higher after insertion of a cemented femoral component than after insertion of a cementless component. Mean pulmonary artery pressures peaked and central venous oxygen tension reached a nadir after reduction of the hip. These measurements attest to the delayed collection of embolic medullary contents in the lung resulting from kinking of the femoral vein during component insertion. Mechanical manipulation while the limb is being positioned for femoral preparation also is likely to cause local intimal injury to the femoral vein, may cause the unique finding of segmental femoral thrombi after THA, and completes an across-the-board disturbance in the Virchow triad (along with hypercoagulability and stasis) in this high-risk scenario.
A subsequent investigation of the efficacy of short-acting anticoagulants for blunting the intraoperative activation of the clotting cascade during THA found that standard heparin administered intravenously after socket implantation significantly inhibited fibrin formation at 10 units/kg and completely suppressed fibrin formation at 20 units/kg (42




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree