INTRODUCTION
Vascular disease includes any condition that affects the circulatory system, encompassing diseases of arteries, veins, and lymph vessels as well as blood disorders that affect circulation. It describes a broad group of clinical conditions ranging from the chronic to the acute and life threatening. The increasing prevalence of peripheral artery disease (PAD) and carotid artery disease among Americans may be related to a rise in the prevalence of diabetes mellitus, just as the increased incidence of varicose veins is linked to rising rates of obesity.
Vascular disease is a nearly pandemic condition that has the potential to cause loss of limb or even loss of life. It manifests as insufficient tissue perfusion that may be acutely compounded by either emboli or thrombi to an existing atherosclerotic condition. Many people live daily with vascular disease; however, in scenarios such as acute limb ischemia, this pandemic disease can be life threatening, requiring emergency intervention to minimize morbidity and mortality.
DIABETIC WOUNDS
ESSENTIALS OF DIAGNOSIS
The lifetime risk of developing lower extremity ulceration in patients with diabetes mellitus approaches 25%.
Comprehensive annual evaluation of the diabetic foot is recommended.
Visual inspection should be performed at every routine visit to the physician.
Although multifactorial in origin, diabetic ulceration is linked primarily to poor blood glucose control.
Diabetic wounds are associated with substantial morbidity and mortality. Diabetes mellitus is a particularly important risk factor in the development of chronic wounds because it is associated with neuropathy, vasculopathy, and immunopathy. Chronic ulceration affects the lower extremities in 1.3% of adults in the United States. However, among diabetic patients the lifetime risk of developing lower extremity ulceration approaches 25%. Two thirds of nontraumatic amputations performed in the United States are secondary to primary diabetic foot ulcers and their complications.
These statistics illustrate the importance of evaluation and prevention of diabetic-related skin infections and the necessity of prompt medical–surgical treatment when infections develop. In 2012, the Infectious Disease Society of America updated its guidelines for the diagnosis and management of diabetic foot infections. The current American Diabetes Association guidelines, which largely agree with those of other organizations, recommend comprehensive annual evaluation of the diabetic foot. This evaluation should include inspection of the foot for the presence of erythema, warmth, and callous, bony, or joint-mobility abnormalities, as well as skin integrity, with time taken to fully evaluate between the toes and under pressure-sensitive metatarsal heads. As part of this evaluation, patients should be tested for loss of protective sensation using tactile, vibratory, and reflex testing, and screened for PAD by asking about claudication symptoms, assessing the pedal pulses, and, in those older than 50 years of age or in any patient having other risk factors of PAD, by assessing the ankle–brachial index (ABI). A visual inspection should also be performed at every routine visit to the physician.
When a patient has a diabetic wound the clinical evaluation should determine the extent and severity of infection, identify the underlying factors that predispose to and promote infection, and assess the microbial cause. The clinical history should note details related to the injury (cause, duration, associated symptoms, and prior treatments, if any). Clinical examination should note the location of the lesion, the presence and extent of infection (local or systemic), the extent of the wound itself (eg, involving superficial skin only or infecting deeper subcutaneous tissues, muscles, bone), and whether bone is visible to the eye or palpable upon probing. Clinical examination should also include a neurologic and vascular evaluation. Laboratory evaluation should include tests to identify systemic or metabolic inflammation (eg, complete blood count, basic metabolic panel, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), as well as a glucose level, to assess glycemic control. Procalcitonin may be a helpful inflammatory marker, but its utility has yet to be proven.
At the biochemical and cellular level, diabetes mellitus involves a complex array of metabolic and vascular factors that shift the balance between nerve fiber repair and nerve fiber damage toward the latter process. The result is a manifestation of polyneuropathy that preferentially affects nerves of the distal extremity. In patients with type 2 diabetes, the vascular factors, which cause ischemia secondary to thickening of the endothelium, are particularly important.
The polyneuropathy involves sensory, motor, and autonomic nerves. Sensory neuropathy diminishes the protective perception of pain that notifies the individual when tissue injury has occurred. Motor nerves to the intrinsic muscles of the foot are affected in approximately half of all diabetic patients, resulting in claw deformities that transfer pressure to the plantar metatarsal heads. The increased tissue pressure may lead to skin erosion and ulceration, and, in the case of the insensate individual, may go unnoticed. Autonomic neuropathy causes the skin to become dry and susceptible to skin fissures, tearing, and infection as a result of loss of sweat and oil gland function. Additionally, diabetes is frequently associated with severe PAD, which affects the smaller distal arteries, causing atherosclerotic changes. The combination of PAD and diabetes contributes to higher rates of nonhealing ulcers and limb loss in diabetic patients compared with nondiabetic patients.
More than 100 known cytologic factors contribute to impaired wound healing in patients with diabetes. These include decreased or impaired growth factor production, angiogenic response, macrophage function, collagen accumulation, epidermal barrier function, quantity of granulation tissue, keratinocyte and fibroblast migration and proliferation, number of epidermal nerves, bone healing, and abnormal balance between the accumulation of extracellular matrix components and their remodeling by matrix metalloproteinases.
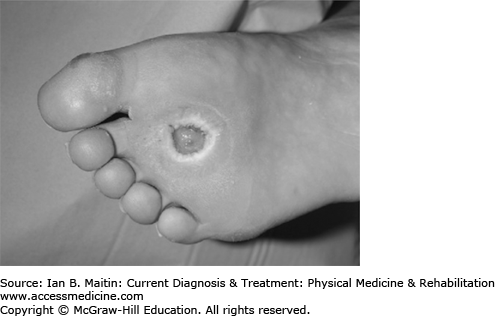
Diabetic ulcers (Figure 3–1) can develop as a result of trauma, skin cracks, fissures, or other defects in the skin of the foot or the paronychia. Infection can be localized to the superficial skin at the site of a preexisting lesion or involve the skin or deeper structures beyond the local site, potentially spreading to joints, bones, or the systemic circulation.
Patients with diabetic foot infections often have universal symptoms of infection (fever, chills, hypotension, and tachycardia) or inflammation (erythema, warmth, swelling, and tenderness), or pustulant material within an ulcer or sinus tract. However, these symptoms are not always noted, as diabetic patients with sensory neuropathy may not experience tenderness, and patients with comorbid PAD may not have excess warmth secondary to ischemia. In such cases, infections may progress to include deeper or systemic systems before a patient seeks medical attention. Other nonspecific symptoms, including nonpurulent drainage, friable or discolored granulation tissue, and undermining of wound edges, may also be present.
Osteomyelitis can occur in a diabetic foot wound with or without evidence of soft tissue infection. Clinical features associated with the presence of underlying osteomyelitis include grossly visible bone or the ability to probe to bone, ulcer size larger than 2 cm2, ulcer duration greater than 2 weeks, and ESR greater than 70 mm/h.
Grading of neuropathy and wounds is equally important in clinical practice. The International Working Group on the Diabetic Foot developed a neuropathy classification system to predict foot ulceration that classifies patients into risk groups (Table 3–1). A commonly used classification system for grading diabetic wounds is shown in Table 3–2.
| Group | Clinical Description |
|---|---|
| 0 | No evidence of neuropathy |
| 1 | Neuropathy present by no evidence of foot deformity or peripheral vascular disease |
| 2 | Neuropathy with evidence of deformity of foot or peripheral vascular disease |
| 3 | History of foot ulceration or lower extremity amputation |
| Grade | Clinical Description |
|---|---|
| 0 | No ulcer in a high-risk foot |
| 1 | A superficial ulcer that involves the full skin thickness but not the underlying tissues |
| 2 | Deep ulcer, penetrating down to ligaments and muscle, but no bone involvement or abscess formation |
| 3 | Deep ulcer with cellulitis or abscess formation, often with osteomyelitis |
| 4 | Localized gangrene |
| 5 | Extensive gangrene involving the whole foot |
In most cases, the history of the infection or wound is sufficient to isolate the source to diabetes. However, other infections or processes can present with generalized inflammatory changes in the skin of the distal extremities and therefore mimic a diabetic infection. Some of these include trauma, crystal-associated arthritis, Charcot arthropathy, fracture, thrombosis, and venous stasis. Other skin ulcerations that manifest similarly to a diabetic ulceration include, but are not limited to, chronic ulcers from venous disease, pressure ulcers, arterial ischemic skin alterations, and localized burn trauma.
Many complications may occur when diabetic infections ulcerate. The most topically important are all manner of infections that if unnoticed, undiagnosed, or untreated can progress to osteomyelitis, potentially requiring aggressive therapy, prolonged hospitalization, vascular intervention, or amputation. Along with the multitude of other impaired functions linked to diabetic wounds, the simple act of ulceration causes the breakdown of the body’s natural barrier mechanism—the skin—allowing bacteria ready access. Most diabetic ulcers, if chronic, are colonized and not necessarily infected. When ulcers do become infected the wounds are therefore polymicrobial.
Superficial diabetic ulcers in most individuals are likely caused by aerobic gram-positive cocci (Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pyogenes, and coagulase-negative staphylococci). Ulcers that are deep, chronically infected, or previously treated with antibiotics are more likely to be polymicrobial. Such wounds may involve the previously mentioned species as well as enterococci, Enterobacteriaceae, Pseudomonas aeruginosa, and anaerobes. Wounds with extensive local inflammation, necrosis, malodorous drainage, or gangrene along with signs of systemic toxicity should be presumed to have anaerobic organisms (Clostridium species, Bacteroides species, and anaerobic streptococci), in addition to the aforementioned organisms.
Considering the predilection of specific organisms to infect diabetic ulcers, further information should be obtained relating to the patient’s the risk of infection with specific organisms. Methicillin-resistant S aureus (MRSA) is a common pathogen, particularly in those who have had previous MRSA infections or known colonization. Other risk factors for MRSA infections are prior antibiotic use, previous hospitalization, and residence in a long-term care facility. P aeruginosa is a prevalent organism in diabetic wounds of patients from warm climates. Macerated ulcers, foot soaking, and other exposure to water or moist environments also increases the risk of P aeruginosa involvement. In the absence of these conditions, it is not a commonly isolated pathogen.
Resistant enteric gram-negative rods (extended spectrum β-lactamase [ESBL] organisms) are increasing in prevalence in diabetic wound infections. Although these bacteria are still uncommon, there is greater risk in patients who have had prolonged hospital stays, prolonged catheterization, prior antibiotic use, or residence in a long-term care facility.
The most important tenet of diabetic wound treatment occurs before an individual develops a wound. Preventive foot care should be routinely discussed with patients who are at high risk for wound development, especially those with existing neuropathy. In conjunction with screening, nutritional support, and medical preventative guidance to control glucose and vascular risk factors, the high-risk patient should be instructed to avoid smoking, walking barefoot, using heating pads, and stepping into the bath without first checking water temperature. Additional guidelines include trimming toenails to the shape of the toe, with the removal of sharp edges; daily foot inspection (by the patient or someone else, if vision or ability is an issue); ensuring proper shoe fit and sock use; and daily bathing of feet in lukewarm water with mild soap, and then blotting dry with a soft towel.
If prevention fails and medical therapy is required for a diabetic wound, multiple treatment strategies can be initiated, all of which have been shown to have positive effects on the healing of diabetic wounds. These include antibiotic treatments, various methods of debridement, assorted topical modalities, wound dressing materials, wound closure techniques, mechanical offloading, and adjunctive therapies.
Antibiotics can be used to treat wounds that are clinically infected. Options range from oral to parenteral coverage, depending on the severity of the wound and the likely bacteria present. The choice of agent should always ensure coverage of species such as S aureus and streptococci (group A and B). Cultures should be obtained to confirm pathogens and, if necessary, alter the antibiotic course. The preferred clinical specimens for reliable culture include aspirate from an abscess or curettage from the ulcer base. Superficial swabs are neither reliable nor sufficient for prediction.
Chronic wounds characteristically have decreased angiogenesis and accumulate devitalized tissue, hyperkeratotic tissue, exudate, and bacterial overgrowth on the surface of the wound, creating a biofilm. Debridement can aide in restoring an optimal wound healing environment and can be done in various ways.
Irrigation with warm, isotonic saline can decrease bacterial load and remove loose material. It should be done with low pressure (< 15 lb psi) as higher pressure could cause local tissue damage by dissecting loose connective tissue and increasing edema.
Sharp, excisional surgical debridement uses a scalpel to remove devitalized tissue and accumulated debris. It functions to decrease bacterial load and stimulates wound epithelialization. Surgical debridement is the most appropriate choice for removing large areas of necrotic tissue and is indicated when there is excessive bone or joint involvement, gangrenous tissue, or any evidence of sepsis.
Enzymatic debridement uses topical proteolytic enzymes such as collagenase, fibrinolysin, and deoxyribonuclease that work synergistically with endogenous enzymes to debride the wound. Autolytic debridement is accomplished by covering a wound so that the endogenous proteolytic enzymes digest the necrotic tissue while the cover prevents external infection. This method should not be used if the wound is infected initially as the topical enzymes will not function as a disinfectant.
Biologic debridement is an alternative method of debridement that utilizes the sterilized larvae of the Australian sheep blowfly (Lucilia cuprina) or the green bottle fly (Lucilia sericata). The larvae (maggots) produce enzymes that degrade necrotic tissue but do not harm healthy tissue. They are kept from migrating by a mesh cover and can remain in place for 48–72 hours before the dressing is changed. The largest disadvantage to this method is the negative perceptions about its use by both patients and staff.
Growth factors and antimicrobials are other alternatives that have been used in diabetic wound care. Platelet-derived growth factor promotes cellular proliferation and angiogenesis. It is indicated for noninfected diabetic ulcers that extend into the subcutaneous tissue and have an adequate vascular supply. Granulocyte–macrophage colony-stimulating factor (GM-CSF) has been used in various chronic wounds to promote healing. Cadexomer iodine is an antimicrobial that reduces bacterial load because it is bactericidal to all gram-negative and gram-positive bacteria. Silver sulfadiazine is a topical antiseptic cream that decreases the incidence of sepsis in cutaneous wounds. It also serves as an antimicrobial, as silver is toxic to bacteria.
Appropriate wound dressings promote ulcer healing by facilitating endogenous mechanisms, absorbing excess exudate, and protecting the wound from the external environment. Wounds that are too wet or too dry heal more slowly; excess fluid causes wound maceration while excess desiccation slows cellular migration to the site. Additionally, as wounds heal the requirement of the wound site changes, so the dressing type should change along with it.
Dressings are classified into three categories—open, semi-open, or semi-occlusive—according to their water-retaining abilities, because maintaining a moist environment is the primary goal in dressing therapy. Open dressings usually consist of gauze, which should never be applied dry. Gauze is inexpensive but requires frequent dressing changes. Semi-open dressings include fine mesh gauze impregnated with some form of ointment; Xeroform and Adaptic dressings are examples. Semi-open dressings are inexpensive but fail to provide good control of exudate or maintain a moisture-rich environment. Semi-occlusive dressings are available with a wide variety of occlusive properties, absorptive capacities, conformability, and bacteriostatic activity. Examples include films, foams, alginates, hydrocolloids, and hydrogels.
An absorptive dressing (eg, alginate, foam, or hydrofiber) should be used for ulcers with heavy exudate. Alginates are derived from brown seaweed and form a gel on contact. Foams provide thermal insulation, high absorbency, and a moist environment; they can be easily cut to shape, do not shed fibers, and are used for exudative wounds with sloughing skin. Desiccated ulcers lack wound fluids, which stimulate epithelialization; thus, a dressing that can provide a moist environment without causing maceration is needed. Saline-moistened gauze, transparent films, hydrocolloids, and hydrogels are commonly used dressings that meet this need. Saline-moistened gauze provides the correct environment but requires frequent dressing changes as it quickly dries out. Additionally, care is needed when changing the dressing to avoid mechanically debriding healthy tissue.
A variety of adjuvant therapies may be employed to aid diabetic wound healing, including negative pressure wound therapy, hyperbaric oxygen therapy, and electrical stimulation.
This therapy, also known as vacuum-assisted closure therapy, relies on subatmospheric pressurized closure of a wound. It has been shown to increase wound perfusion, reduce edema, and reduce the local bacterial burden while increasing the formation of granulation tissue.
Hyperbaric oxygen increases mobilization of endothelial progenitor cells, which have been shown to aid healing by initiating angiogenesis in a hypoxic wound. The technique has shown utility in preventing amputation and improving wound healing. However, the therapy cannot be targeted to the wound site, and systemic exposure has been associated with serious adverse events, including seizures and pneumothorax.
Direct electrical current applied to a wound promotes migration and proliferation of fibroblasts. Electrical stimulation has been shown to enhance healing during the initial stages of wound closure.
Pressure modulation is a key intervention in the treatment of diabetic ulcerations, especially in the lower extremities. Thus, the concept of mechanical offloading is of pivotal importance when considering therapeutic options for the diabetic foot.
The biomechanical causes of foot ulceration are pressures that are applied to the foot during stance phase. The ground reaction forces generated in response to weight bearing contain vertical, anteroposterior, and mediolateral components, with the vertical aspect much greater than the other two. (For additional discussion of this topic, see Chapter 4.) The vertical force created during a fast walk phase peaks at approximately 1.5 times body weight. While running, this force increases to approximately 5 times body weight. Vertical force damages tissue by compressing and deforming it. The anteroposterior and mediolateral forces jointly create a shear that damages healthy tissue by stretching it. It is the combination of focal pressures and repetitive stress applied to the plantar aspects of the diabetic foot that results in foot wounds.
Pressure disbursement is most successful when the pressure forces are spread over a wide area or, as possible, eliminated. Common offloading methods include bed rest, wheelchairs, crutches, and the use of orthotics. Although bed rest, wheelchairs, and crutches are commonly prescribed they may precipitate other problems, hurting more than they help. They require the patient to limit full use of the lower extremities, which can lead to muscle loss, contraction, decreased endurance, and other immobility-related problems. They also shift pressure to other body areas, which can cause additional pressure-related injuries. Crutches exert pressure under the arms and place greater pressures on the contralateral high-risk diabetic limb; use of beds and wheelchairs increases pressure on the coccyx and ischial spines. Often patients do not have the endurance or strength to use crutches, or their homes cannot accommodate the bulkiness of a wheelchair. For all these reasons, correct use and compliance with these options has been shown to be quite poor. (These and other issues relating to use of specific assistive devices are discussed in more detail in Chapter 41.)
Prescribed orthotics to the lower limb have shown much better results in wound healing; they include half-shoes, healing sandals, bespoke (custom-made) therapeutic shoes, removable cast walkers (RCWs), total contact casts (TCCs), Scotchcast boots, and instant total contact casts (iTCCs).
Half-shoes were developed to reduce postoperative pressure on the forefoot. The shoe consists of a wedged sole that ends just proximal to the metatarsal heads, eliminating propulsive gait and decreasing ground forces applied on the forefoot. They are inexpensive and easy to apply.
Healing sandals are specially designed to limit plantar progression of the metatarsal heads during propulsive gait by limiting dorsiflexion of the metatarsophalangeal joints through the application of a rigid rocker to the sole. They are lightweight, stable, and reusable. However, they are expensive and the rocker sole requires a significant amount of time and experience to produce.
Bespoke therapeutic shoes are a commonly prescribed customized shoe built with measurements taken from the patient. Although they have not shown utility in helping wounds heal, they are very effective in preventing ulcerations in high-risk feet. Because they are custom made, they can be quite expensive and patients may not find them to be aesthetically pleasing.
RCWs are castlike devices that are removable to allow for self-inspection of the wound and application of topical therapies that require frequent administration. RCWs are indicated for infected ulcers as well as superficial tissue infections. They limit propulsion by keeping the ankle at 90 degrees, thereby helping to decrease forefoot plantar pressure. Patients report better ease in sleeping and bathing as they can take the devices off during these activities; however, they can also remove the device inappropriately. Removability is the best feature of the RCW; it is also paradoxically its worst. The ability to remove the RCW eliminates “forced compliance” in use of the device, which makes TCC the gold standard in achieving pressure distribution.
TCC systems employ a plaster-of-Paris, well-molded, minimally padded cast that maintains contact with the entire plantar aspect of the foot and the lower leg. Intimate fit to the plantar surface of the foot increases the weight-bearing surface area. TCCs are indicated in the treatment of noninfected and nonischemic plantar diabetic wounds, with reported healing rates that range from 72% to 100% over a 5- to 7-week course. They have been shown to reduce pressure at the site of ulceration by 84–92%, reduce inflammatory and reactive components that detract from the reparative process, and reduce or control edema that impedes healing. Because the cast is a solid entity, it prevents exposure to foreign objects and pathogens. Most importantly, however, it ensures patient compliance because the cast is immobile and nonremovable.
The TCC system is not without disadvantages, some of which may preclude prescription of this modality. Application of the cast is time-consuming and requires a trained cast technician. Improper application—the most significant detractor—can cause skin irritation and, in extreme cases, new ulceration. Because the TCC (unlike the RCW) cannot be removed, the wound cannot be assessed frequently. TCCs are therefore contraindicated in patients with soft tissue infections or osteomyelitis. Patients have also reported difficulty sleeping and bathing because of the precarious immobility of the 90-degree ankle, and the necessity of keeping the cast dry.
Scotchcast boots are an alternative to plaster-of-Paris boots created with much lighter, stronger, and more durable fiberglass polymers. The Scotchcast boot acts much like a TCC and shows similar healing rates, although it is more expensive to create. Small studies have reported fewer complications with the fiberglass material than the plaster-of-Paris TCCs.
Construction of an iTCC device involves simply taking an RCW as a base and wrapping it with bandage, Elastoplast, or casting tape. The ability to remove and reapply the device—but not without effort—addresses both the compliance issues associated with RCWs as well as the limitations of the standard TCC with regard to wound management. The first randomized controlled study comparing the iTCC with the TCC found no differences in healing rates or complications between the two and showed that the cost of materials and personnel was much lower with the iTCC. A parallel study comparing healing rates of the iTCC with the RCW showed that the iTCC was superior.
Diabetes is the underlying cause of most nontraumatic lower extremity amputations in developed countries, and infection is the precipitating event for nearly 90% of these amputations. Research has shown that with increasing infection severity there is a clear trend toward increased risk for amputation, increased risk for more proximal anatomic amputation, and increased need for lower-extremity-related hospitalizations. The same research revealed a similar trend toward increasing risk for experiencing other diabetic foot-related complications, such as neuropathy or vascular disease. This suggests that persons with noninfected or mild wounds are highly unlikely to require hospitalization, develop osteomyelitis, or undergo amputation, whereas those with moderate to severe wounds require much more diligent, thorough, and expansive treatment strategies that all too often result in amputation.
PERIPHERAL ARTERY DISEASE
ESSENTIALS OF DIAGNOSIS
Commonly caused by atherosclerosis.
The initial symptom is usually intermittent claudication, but patients are often asymptomatic.
Associated with an increased risk of cardiovascular mortality.
Angiography is the gold standard for diagnosis.
PAD describes the condition of impaired blood flow to the extremities, most commonly caused by atherosclerosis. Depending on the degree of arterial stenosis and collateral circulation, PAD can lead to varying clinical presentations. However, in a significant segment of the PAD population, possibly up to 75%, presentation is asymptomatic. PAD prevalence increases significantly with age and is more common in non-Hispanic blacks. Men and women develop PAD at similar rates, but it may be slightly more common in men. The most important risk factors that have been repeatedly associated with PAD are smoking, diabetes mellitus, hypertension, and hyperlipidemia. PAD is also more prevalent in patients with chronic renal insufficiency, hyperhomocysteinemia, hyperviscosity, and hypercoagulable states. A self-reported history of cardiovascular disease, including coronary artery disease, congestive heart failure, and cerebrovascular accidents, has been strongly correlated with PAD.
PAD is often asymptomatic, but when blood flow limitation becomes significant the first symptom is usually intermittent claudication. Intermittent claudication produces leg pain, numbness, or fatigue during exercise and subsides with rest. The symptoms can occur at different levels of the lower extremity, depending on where the arterial stenosis is located. The most common site of PAD is the superficial femoral artery, leading to intermittent claudication of the calf muscles. PAD can also occur in the iliac, common femoral, and tibioperoneal arteries, causing symptoms in the buttocks, thighs, and feet, respectively.
Individuals with intermittent claudication have dysfunctional oxidative metabolism in the affected muscles. This dysfunction leads to skeletal muscle injury, with loss of muscle fibers and atrophy. As a result, these patients have a lower tolerance for physical activity than those without PAD and an associated decline in the ability to perform activities of daily living. Patients often develop collateral circulation or alterations in their gait pattern suggestive of disease stabilization, but thorough evaluation reveals symptoms that are usually slowly progressive, leading to a decline in pain-free walking distance. About 25% of patients with intermittent claudication progress to rest pain or critical limb ischemia, manifested by ulceration and gangrene. Patients with rest pain frequently describe hanging their legs over the edge of the bed or sleeping in a chair to increase distal perfusion and relieve symptoms. Fontaine first classified this progression from asymptomatic PAD to critical limb ischemia (Table 3–3).
PAD is associated with a three- to six-fold increased risk of cardiovascular mortality. Patients with lower ABI values (indicating more severe PAD) and those with abnormally elevated ABI values (indicating arterial calcifications) have a higher risk of cardiovascular events. Therefore, physicians should pay close attention to any complaints of chest pain, angina, and related symptoms in these patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree