Fig. 2.1
Limited joint mobility.
Flexion contracture of the metacarpophalangeal and proximal interphalangeal joints in fingers
Table 2.1
Rheumatic complications of DM
Conditions unique to DM |
Diabetic muscle infarction |
Conditions occurring more frequently in DM |
Limited joint mobility |
Dupuytren’s contracture |
Stenosing flexor tenosynovitis (trigger finger) |
Adhesive capsulitis of shoulder |
Carpal tunnel syndrome |
Neuropathic arthropathy |
Conditions sharing risk factors of DM and metabolic syndrome |
Diffuse idiopathic skeletal hyperostosis |
Osteoarthritis |
Gout |
Pseudogout |
Calcific periarthritis of shoulder |
Osteoporosis |
Sarcopenia |
2.2 Dupuytren’s Contracture
Dupuytren’s contracture is characterized by palmar or digital thickening, tethering, pretendinous bands, and flexion contractures of the fingers (Fig. 2.2). In patients with diabetes, both genders are equally affected, and the ring and middle finger are more commonly involved, while in nondiabetic patients, men are more likely affected, and the small finger is more involved. The prevalence of Dupuytren’s contracture in diabetic patients ranges between 16 and 42 % [8, 9] compared with 13 % in the general population [10]. The prevalence is higher in patients who are older and with a longer duration of DM. Patients with Dupuytren’s contracture should be evaluated for DM, as 13–39 % of them are found to have DM [11]. The pathophysiology of Dupuytren’s contracture is likely to be multifactorial. Genetic predisposition explains the higher prevalence in some population over others. Trauma, long-term hyperglycemia, microangiopathy, and ischemia are also important factors. Microvascular disease and ischemia will result in increased production of oxygen free radicals. Ischemia also stimulates platelets and macrophages to produce different cytokines such as interleukin 1, tumor necrosis factor, and growth factors, such as platelet-derived growth factor, epidermal growth factor, connective tissue growth factor, vascular endothelial growth factor, and basic fibroblast growth factor, resulting in local collagen overproduction and fibrosis [12].


Fig. 2.2
Dupuytren’s contracture in the small finger characterized by palmar thickening, tethering, pretendinous bands, and flexion contractures
Treatment consists of optimizing glycemic control and physiotherapy. Recently, injections of collagenase clostridium histolyticum have been developed for the treatment of Dupuytren’s contracture. Hurst et al. in 2009 [13] did a prospective, randomized, and placebo-controlled trial of 308 patients with Dupuytren’s contracture, 6.5 % of whom had DM; up to three collagenase injections significantly reduced fixed flexion contractures and improved the joint range of motion. There were no recurrences after follow-up of up to 90 days. Only three serious adverse events were reported in this study, including two tendon ruptures and one case of complex regional pain syndrome. Surgery is required if the hand function is severely compromised.
2.3 Stenosing Flexor Tenosynovitis (Trigger Finger)
Flexor tenosynovitis is caused by fibrous tissue proliferation in the tendon sheath (Fig. 2.3) leading to limitation and restriction of the movement of the tendon, also known as trigger finger. The prevalence of flexor tenosynovitis is estimated at 11 % in diabetic patients, compared with less than 1 % in nondiabetic individuals [14]. Patients with DM are more likely to have multiple digit involvement. It most commonly involves the ring, middle fingers, and thumb. The occurrence of flexor tenosynovitis correlates significantly with the duration of DM, but not with glycemic control [15]. Treatment of flexor tenosynovitis includes modification of activities, splinting, NSAIDs, corticosteroid injection into the tendon sheath, and surgical release [16].
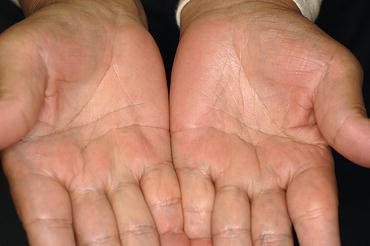

Fig. 2.3
Stenosing flexor tenosynovitis in the right small finger and the left ring finger characterized by fibrous tissue proliferation in the palmar tendon sheaths
2.4 Carpal Tunnel Syndrome
Carpal tunnel syndrome (CTS) is an entrapment neuropathy caused by compression of the median nerve within the carpal tunnel. It is estimated to occur in 3.8 % of the general population [17]. The prevalence of CTS in diabetic patients is higher and estimated to occur in 14 % of patients without diabetic polyneuropathies and up to 30 % in those with diabetic polyneuropathies [18]. CTS prevalence increases as the DM duration extends. Increased prevalence of CTS in diabetes suggests the presence of intrinsic nerve pathology in addition to mechanical compression. The intrinsic nerve factors include loss of normal regenerative ability in the peripheral nerve because of microangiopathy, macrophage dysfunction, abnormalities in the retrograde cell body reaction, Schwann cell dysfunction, or decreased expression of neurotrophic factors and their receptors [19]. CTS manifests as pain, tingling, and paresthesia of the thumb, index, middle fingers, and the radial aspect of the ring finger. The symptoms may be improved by shaking or flicking the wrists known as “flick sign.” A reduction of the pinch strength caused by the atrophy of the thumb ball and function of the affected hand may occur (Fig. 2.4). Symptoms tend to worsen at night. Bilateral CTS is common, but the symptoms may not occur simultaneously in both hands. It is usually diagnosed by history and clinical examinations, by percussion of the median nerve at the wrist (Tinel’s test), by asking the patient to do wrist dorsiflexion (Phalen’s test), or by employing the hand elevation test which is conducted by asking the patient to raise the affected hand and holding it in that position for 1 min. The test is considered positive if the patient experiences tingling and numbness in the median nerve distribution area [20, 21]. The diagnosis of CTS is confirmed by a nerve conduction study. Imaging studies, including magnetic resonance imaging (MRI) and ultrasonography (US), can be useful for this purpose. US is a simple, easy-to-perform, and noninvasive procedure. It has been implicated in the diagnosis of CTS as it can demonstrate the thickening of the median nerve, the flattening of the nerve within the tunnel, and the bowing of the flexor retinaculum, which are all features that indicate the presence of CTS. Several studies have concluded that the cross-sectional area is the most predictive measurement, but there is debate regarding the level within the tunnel that this measurement should be taken and what constitutes abnormal values. The sensitivity of US is 64.7 % [22]. An MRI will demonstrate swelling of the median nerve and increased signal intensity on T2-weighted images, indicating accumulation of the axonal transportation, myelin sheath degeneration, or edema, which are the signs to look out for when diagnosing CTS. An MRI shows the severity of the nerve compression and has a sensitivity of 96 %. However, its specificity is extremely low at 33–38 % [22]. The treatment options for CTS include splinting and local injection of corticosteroids or NSAIDs. Although corticosteroid treatment is effective in reducing inflammation and edema, it limits the tenocyte function by reducing collagen and proteoglycan synthesis, thus reducing the mechanical strength of the tendon and ultimately leading to further degeneration [23]. When conservative treatment fails, surgery is indicated. Surgery is performed more frequently among patients with DM and is estimated to be 4–14 times higher than the general population [24].
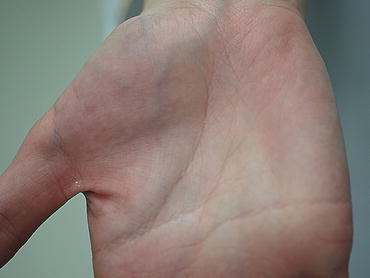

Fig. 2.4
The atrophy of the thumb ball due to median nerve palsy caused by carpal tunnel syndrome
2.5 Adhesive Capsulitis of the Shoulder (Frozen Shoulder)
Adhesive capsulitis, or frozen shoulder, or shoulder periarthritis is characterized by progressive painful restriction of the shoulder movements, especially the external rotation and abduction. The prevalence in diabetic patients ranges 10–29 % [5, 16], as compared with 3–5 % of the age-matched controls [25]. The presence of shoulder adhesive capsulitis increases the incidence of DM and may be a presenting symptom. Connie et al. in 2008 studied the prevalence of a diabetic condition (DM and prediabetes) and adhesive capsulitis of the shoulder which revealed that a patient presenting with adhesive capsulitis had a 71.5 % chance of having a diabetic condition (38.6 % chance of being diabetic and a 32.95 % chance of being prediabetic) [26]. The natural history of the condition is characterized by three phases: pain, adhesion or stiffness, and recovery phases. Among diabetics, it occurs at a younger age, is less painful, lasts longer, and responds less well to treatment [27]. Bilateral involvement is more frequent in patients with diabetes than in nondiabetic subjects (33–42 % vs. 5–20 %) [28]. It occurs more in older patients with longer disease duration. Shoulder adhesive capsulitis is found to be associated with other diabetic complications such as limited joint mobility, autonomic neuropathy with either type of DM, and with myocardial infarction in patients with type 1 DM [5]. The exact mechanism is unknown, but it is thought that excessive glucose concentration in diabetic patients can lead to a faster rate of collagen glycosylation and cross-linking in the shoulder capsule, restricting shoulder range of motion [29]. Management of shoulder adhesive capsulitis in the painful early phase consists of adequate analgesia, intra-articular corticosteroid injections, and an appropriately graded exercise program. During the adhesive phase, physical therapy and operative treatments (arthroscopic capsular release or open surgical release) are used.
2.6 Diffuse Idiopathic Skeletal Hyperostosis
Diffuse idiopathic skeletal hyperostosis (DISH) is a condition that is characterized by diffuse calcification and ossification of the ligaments and enthesis (Figs. 2.5 and 2.6). The prevalence of DISH in type 2 DM was reported at 13–40 %, while in general population, 2.2–3.5 %. The prevalence of metabolic syndrome is higher among patients with DISH [16]. It most commonly affects the spine, particularly the thoracic spine. Patients with DISH are rarely symptomatic. However, it can cause spinal rigidity and impingement of nearby structures and nerves, resulting in hoarseness, stridor, sleep apnea, and dysphagia. It also can occur in extraspinal sites with prominent bony reactions at ligamentous and tendinous insertions, particularly in the pelvis, greater trochanters, patellae, and calcaneus. The diagnosis of DISH is based on radiologic features that are usually based on Resnick and Niwayama’s 1976 criteria: (1) flowing ligamentous calcifications involving at least four contiguous vertebral bodies, (2) preservation of intervertebral disk space, and (3) absence of changes of degenerative spondylosis or spondyloarthropathy. The exact mechanism of DISH is not known. However, insulin, growth hormone, and growth factor (IGF-1) are proposed as factors that promote bone growth in DISH. Also, atherosclerosis, which is common in metabolic syndrome, will lead to damage in the endothelium and aggregation of platelets that result in increased levels of IGF-1 and then more osteoblast proliferation and bone formation. The treatment of DISH is symptom based and generally limited to analgesia as needed. Rarely, surgical removal of impinging bone bridges is undertaken when critical functions, such as swallowing, are compromised.
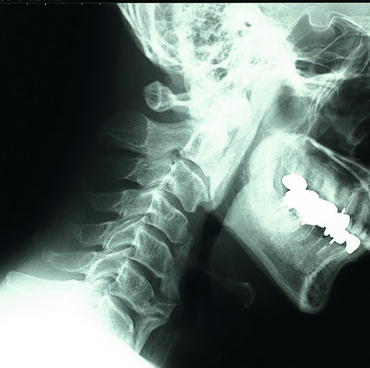
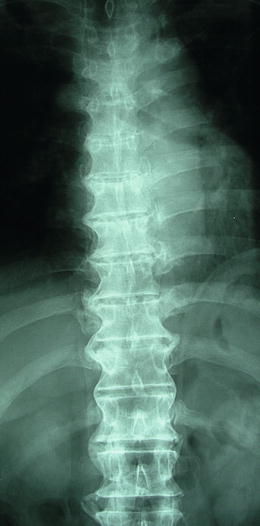

Fig. 2.5
The anterior longitudinal ligamentous calcifications of cervical vertebrae in diffuse idiopathic skeletal hyperostosis

Fig. 2.6
The ligamentous calcifications of thoracolumbar vertebrae in diffuse idiopathic skeletal hyperostosis characterized by no lesion in heart side of thoracic vertebrae
2.7 Osteoarthritis
Osteoarthritis (OA) is a very common form of arthritis in adults. Several risk factors are described for OA, including obesity, which is part of metabolic syndrome and not uncommon in DM. Dahaghin et al. [30] described an association between diabetes and hand osteoarthritis that was noted in people aged 55–62 years and was absent in other age groups. Peripheral neuropathy may increase the risk of advanced, aggressive forms of osteoarthritis. Recently, an association between knee osteoarthritis and diminished lower extremity vibratory perception was identified [31]. However, there is no clear evidence that supports DM or metabolic syndrome as risk factors for developing early or severe hip or knee OA [32].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







