Bone is the second most commonly implanted material in the human body, after blood transfusion, with an estimated 600,000 grafts performed annually. Although the market for bone graft substitutes is more than $1 billion, that of bone graft itself is still more than half that amount. Reports of autologous bone grafting date back to the ancient Egyptians, yet the modern scientific study of grafting began in the early 19th century. Since then, the indications, methodology, and science of bone grafts in nonunion and bone loss have been established and refined, and new methods of harvesting and treatment are being developed and implemented. This article describes the use of solid and cancellous bone graft in the treatment of acute bone loss and nonunion.
Bone is the second most commonly implanted material in the human body, after blood transfusion, with an estimated 600,000 grafts performed annually. Although the market for bone graft substitutes is more than $1 billion, that of bone graft itself is still more than half that amount. Reports of autologous bone grafting date back to the ancient Egyptians, yet the modern scientific study of grafting began in the early 19th century. Since then, the indications, methodology, and science of bone grafts in nonunion and bone loss have been established and refined, and new methods of harvesting and treatment are being developed and implemented. This article describes the use of solid and cancellous bone graft in the treatment of acute bone loss and nonunion.
The modern study of bone grafting can traced back to the work of Ollier in the mid-1800s, when he showed that transplanted bone could be osteogenic (although some earlier work examined the osteogenic qualities of bone). Nearly 50 years later in 1914, Phemister showed what occurs to a graft when transplanted. He found that some of the transplanted cells survive at the endosteal or periosteal surface, but that the deeper cells die. Also, over time older bone is replaced by newly formed bone. This process, the remodeling by osteoclastic resorption and creation of new vascular channels with osteoblastic bone formation, would progress to become what is now defined as creeping substitution .
In other clinical studies, Phemister described a novel technique with excellent results of onlay bone grafting, in which a pocket is created around the recipient site through lifting an osteoperiosteal sleeve with slivers of cortical bone. The graft is placed within this created space and the ununited fracture callus is left undisturbed, the latter concept going against the prevailing practice at the time ( Fig. 1 ).
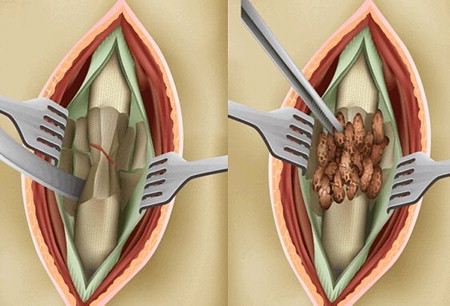
Judet and Judet described another method for exposing onlay bone graft to vascularize host tissue, wherein the cortical surface of the bone was elevated with an osteotome in what is best described as fish scaling . In this method, the outer cortex is lifted to expose the graft to underlying haversian canals and cortical capillary bleeding ( Fig. 2 ).
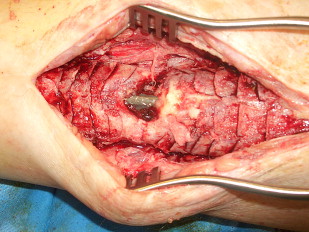
Further advancing the technique of bone grafting, in 1945 Harmon described the technique still commonly used today of posterolateral grafting for nonunion of the tibia. The goal was to create a synostosis between the tibia and the fibula. This method provided considerable advantage over previous methods. Through placing the graft posterior, potentially compromised soft tissue or infection is avoided anteriorly, and the improved vascular supply of the posterior musculature leads to faster graft incorporation ( Fig. 3 ).
The understanding of bone graft physiology advanced significantly with Urist’s work in 1965 when he showed the capacity of devitalized bone matrix to induce bone formation in heterotopic sites. In further experimentation, Urist and colleagues identified bone morphogenic protein (BMP), a substance they believed was capable of signaling the differentiation of mesenchymal cells into cartilage and bone. Studies have now shown more than 40 different BMPs. The commercially available BMP products (BMP2 and BMP7) are in wide clinical use and discussed elsewhere in this issue.
Today, experts understand that Urist’s early work identified the processes of osteoinductivity, osteoconductivity, and osteogenicity. Osteoinduction is the process through which a signal is sent to influence the formation of new bone. The aptly named BMPs have been shown to play a significant role in this process, but many other molecules and proteins (eg, transforming growth factor beta) are involved in the process. Osteoconduction refers to the scaffolding over which new bone must grow for healing to occur. The properties of the scaffold, such as material, pore size, and porosity, can influence the rate of incorporation of a bone graft. Osteogenesis is the graft’s ability to form bone by way of its cellular elements, whether through differentiation of mesenchymal cells or recruitment of osteoblasts and osteocytes. Autologous bone graft remains the gold standard because it is believed to contain all three characteristics.
Fundamental to the discussion of bone graft is an overview of bone formation, fracture healing, and graft incorporation. Bone is formed in one of two ways. Endochondral ossification involves the creation of a cartilage model of bone that is secondarily used by osteoblasts as a template for bone formation. The steps are chondrocyte proliferation, chondrocyte hypertrophy, matrix mineralization, apoptosis, vascular invasion, ossification, and remodeling to lamellar bone. This process is how long bones are formed.
Intramembranous ossification avoids the use of a calcified cartilage template and relies on the direct formation of bone matrix by osteoblasts. This process is responsible for the formation of flat bones and for the growth in width of the long bones.
Fracture healing involves both types of bone formation to varying degrees, depending on stability at the fracture site. High stability results in direct osteonal healing (also known as direct or primary ) bone formation, which is similar to intramembranous healing. In the setting of lower stability (motion at the fracture site), endochondral (also known as indirect or secondary ) bone formation occurs. In vivo, fracture healing typically involves a combination of these processes.
A major consideration in healing and bone grafting is the viability and vascularity of the tissue bed, because the recipient site plays a major role in determining the success of grafting. The results of bone healing and grafting in various anatomic sites reflect this concept. The femur and forearm uncommonly have healing problems because they have a well-vascularized muscle envelope. The distal tibia, however, remains the nemesis of the fracture surgeon.
Aside from the limb segment, the anatomic site within a given bone, whether diaphyseal or metaphyseal, will also influence the needs of a graft. A contained metaphyseal defect is vascular and surrounded by host bone, with a greater surface area to which a graft can consolidate. The contained defect, through exposure to local host bone, will also have access to local bone cells, making recruitment of osteogenic cells easier.
A diaphyseal defect is usually uncontained and may not even have a healthy muscle envelope at its margins. Therefore, any graft placed into such a hostile environment will first require a potent angiogenic influence, followed by a chemotactic element to recruit appropriate osteogenic cells before bone formation. The authors’ decision algorithm for bone graft use centers on the viability of the tissue bed, nature of the defect, and structural needs.
A contained metaphyseal defect does not generally need potent graft material such as autograft, and can be well treated with alternative methods such as allograft or synthetic cements. Conversely, in segmental bone loss, which has poor vascularity and osteogenic potential, an autogenous bone graft remains the gold standard.
Nonunions can be subdivided into hypertrophic, oligotrophic, and atrophic types, which guides the needed intervention to achieve healing. Hypertrophic and oligotrophic nonunions are similar in that they have biologic potential, with a blood supply sufficient to allow for healing ( Fig. 4 ). Although the former is usually caused by a lack of fracture stability and is characterized by large amounts of callus formation, the latter exhibits little callous at the fracture site, often because of poor fracture reduction or inadequate bone contact.
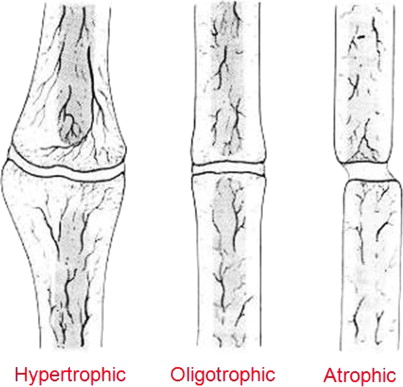
Hypertrophic nonunions require stability, and when this is provided, they achieve bony union quickly, without the need for any bone grafting or biologic augmentation. Oligotrophic nonunion treatment frequently involves improving fragment alignment, with bone grafting performed selectively, depending on the condition of the bone bed and surrounding soft tissue envelope. If the bone and soft tissue envelope are disrupted during the process of achieving appropriate alignment and stabilization, then bone grafting should be considered because an iatrogenic lowering of the biologic potential has occurred. If the alignment and stabilization can be achieved biologically, then bone grafting could be optional and depends on the judgment of the surgeon.
Atrophic nonunions are an entirely different entity. They lack a sufficient blood supply and are located within a zone of injury that frequently has other damaged structures. The atrophic nonunion is lacking biologic potential and requires bone grafting, with the autograft still the gold standard. In cases that also require structural support, cortical autograft can be used, but in many of these situations, surgeons may choose to use a structural allograft or synthetic core with a surrounding biologic autograft to encourage a bridging osseous connection. In the atrophic nonunion and potentially in the oligotrophic nonunion, the decortication methods described previously are needed to stimulate a new healing response from the local host bone and expose the graft bone to a bleeding osseous surface.
The discussion of autologous grafting for acute bone loss and nonunion would not be complete without the mention of distraction osteogenesis. Distraction osteogenesis was pioneered by the Russian Gavril Ilizarov in the 1960s. He found that bone can form new bone when it is gently distracted by up to 1 mm/d. Large bone defects have been successfully treated with this method. In general, the phenomenon of distraction osteogenesis is most similar to intramembranous ossification, and although some unique differences have been noted, their discussion is not within the scope of this article. Unfortunately, the tissue that forms between distracted bone ends is soft and requires a period of secondary support to allow the regenerated bone to mature. This method of autograft, although having distinct disadvantages and morbidity for other reasons, does not have much donor site morbidity, is an excellent form of autograft, and may actually be the ultimate autograft.
Another method of autologous grafting involves the use of bone marrow aspirates. Although technically not bone, the graft is generally obtained from the pelvis to obtain the same autologous inductive proteins and osteogenic cells found in pelvic autografts. Both osteogenic and osteoinductive cells and factors are present in the marrow aspirate. Therefore, theoretically, if they are combined with allograft or similar bone graft substitute, the resulting combination should be similar to autogenous cancellous graft.
This theoretical claim has not been shown in scientific clinical studies. One possible reason is that autogenous bone itself may harbor certain elements that allogenic bone does not (lost during its procurement and processing). Additionally, bone marrow aspirate contains a variable number of osteoprogenitor cells depending on several factures, including age, sex, and harvesting technique.
Studies that have promoted bone marrow aspirate for nonunions described their use in stable nonunions, implying that the nonunions were hypertrophic or oligotrophic. By virtue of being a stable nonunion, they may potentially have healed without the use of bone marrow aspirate and therefore may not be extrapolated for use in an atrophic nonunion. Furthermore, most nonunion sites do not contain readily created spaces around the bone and are usually surrounded by dense fibrous tissue. They require the creation of a potential space around the site, and would also benefit from some type of surface preparation of the site to facilitate an interaction between the marrow elements and local host tissue. The authors have not found bone marrow aspirate alone to be useful, but believe it may be a complementary ingredient when combined with other factors during an open procedure.
The technique of harvesting bone marrow aspirate is important to optimize osteogenic element, otherwise most of the aspirate may contain only hematopoietic elements that would not be useful for bone healing. A recent study showed an average of 1 in 20,000 cells, with a range of 1 in 5000 to 1,000,000. After the first 4 mL, essentially zero osteoprogenitor cells were found in the aspirate.
Finally, another newer source of autogenous graft is the medullary canals of the femur and tibia, using the reamer irrigator aspirator. This subject is discussed in detail in articles found elsewhere in this issue.
Donor sites
The iliac crest has been and is still the most common area for obtaining autogenous cancellous bone graft because of the relative ease of access and the quantity of graft that may be obtained without compromising bone integrity to skeletal support. Iliac crest bone graft also contains all elements recommended for bone graft: osteogenic cells, an osteoconductive scaffold, and osteoinductive proteins. Graft may be obtained from the posterior or anterior ilium, a decision generally determined by patient positioning: posterior grafts are obtained when the patient is prone, whereas the anterior approach is used with the patient supine. Harvesting of the graft is accomplished by opening a cortical window and removing cancellous bone. Studies have reported volumes of around 50 mL from anterior and posterior sites.
When using the posterior ilium, the posterior superior iliac spine is palpated and an incision is made centered around this structure, being mindful to not extend beyond 8 cm from the posterior superior iliac spine, where the cluneal nerves are at risk. Additionally, the ligamentous structures of the sacroiliac joint (medial) and the sciatic foramen (distal) should be avoided to prevent sacroiliac instability or damage to the sciatic nerve and the superior gluteal artery. Approaching anteriorly, the incision should extend along the anterior ilium, stopping 1 to 2 cm lateral to the anterior superior iliac spine to avoid the lateral femoral cutaneous nerve and inguinal ligament.
Although generally regarded as safe, complications and morbidity associated with iliac crest bone grafting have been reported, mostly with anterior crest harvest sites. Wound complications can occur in approximately 21% of these grafts when incisions are made directly over the crest, but far fewer when the incision is made superior or inferior to the crest. Nerve injury can occur to the lateral femoral cutaneous nerve, which has a variable course but most frequently exits anterior and inferior to the anterior superior iliac spine.
Although anesthesia from transaction of the lateral femoral cutaneous nerve is minimal and well tolerated, painful neuromas can result in a condition called meralgia paresthetica . The authors believe that in pelvic and acetabular surgery, it is better to surgically transect the nerve rather than risk a stretch injury, which could lead to meralgia paresthetica.
Blood loss is also expected with iliac crest bone grafting. Arterial injury and hemorrhage is rare, although reported with posterior grafting and violation of the sciatic notch. Hematoma formation, which has been described in as high as 4% to 10% of patients, can be decreased with topical hemostatic agents, and suction drainage. The hematoma and any subsequent drainage can also rarely lead to infection. Arteriovenous fistula and ureteral injury can occur with posterior iliac crest harvesting, although these are limited to case reports. Less commonly, gluteal gait from abductor weakness can occur from excessive stripping of the outer table.
Pain is probably the most common complaint after iliac crest bone grafting. Laurie reported that all patients had moderate pain at 6 weeks, and 10% experienced pain beyond 2 years. Goulet and colleagues found 38% had pain extending to 6 months and 18% experienced pain at 2 years postoperatively.
Unfortunately, although most pain seems to fade with time, chronic pain is likely an unavoidable result in a small percentage of patients. Because some reports indicate that the incidence of pain may be less than the 38% reported by Goulet and colleagues, some technical factors may be associated with this particular morbidity, and attention to surgical technique and soft tissue handling may minimize this morbidity.
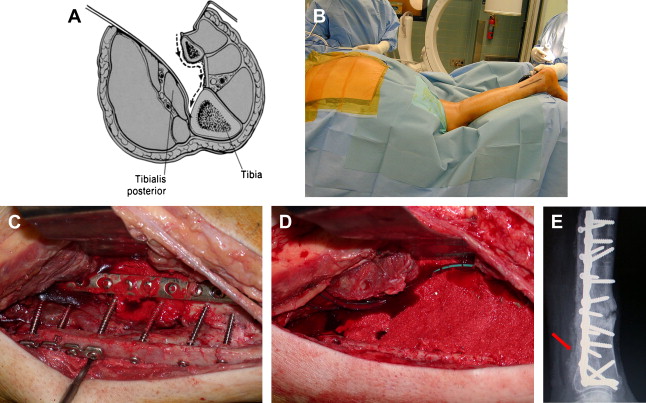
The authors have found that when significant lengths of crest have been harvested (as in tricortical grafts), reconstructing the arc of the iliac wing is helpful to prevent muscle herniation and painful belt wear. Many techniques and products exist for this purpose, but the authors have found that use of a curved 3.5-mm pelvic reconstruction plate across the gap is helpful and, unless incompetence of the iliacus muscle is present, visceral herniation is rare. Still, this complication is rare and serious, as shown in ( Fig. 5 ).
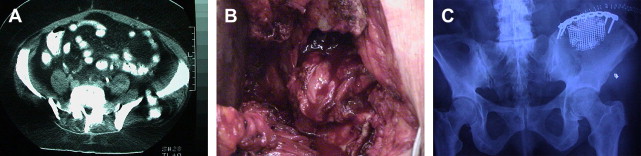
In an attempt to avoid the morbidity of iliac crest harvesting, other sites and techniques have been developed. Westrich and colleagues described a method of harvesting using an acetabular corticocancellous reamer, which they found to be safe and efficacious compared with traditional methods. They note an advantage in the amount of graft obtained (three reamer cups full), but fail to provide a defined quantity.
Sanders and DiPasquale described a similar technique using an acetabular reamer, but in the posterior pelvis. Similarly, copious amounts of graft are reported to be available but not quantified. These investigators reported less discomfort while obtaining large amounts of graft.
The tibia and distal femur have been described as a site for obtaining autogenous cancellous bone graft. The distal tibia is commonly used in foot and ankle surgery because of the proximity to the surgical site and also because small amounts of graft are needed in these procedures. in 1991. O’Keeffe described the proximal tibial metaphysis as an alternative to the iliac crest. Reports have shown that up to 30 mL of graft can be obtained from the proximal tibial metaphysis. Krause and Perry proposed the distal femur as a graft site, providing an average quantity of 10.4 g. They also acknowledge the devastating potential of a supracondylar femur fracture, but note that with a 6-week non-weightbearing period, patients experienced no major complications.
Recipient sites
In the forearm, Wright and colleagues retrospectively investigated the acute cancellous bone grafting in radius and ulna fractures. They found union rates to be comparable in grafted and ungrafted individuals treated with open reduction and internal fixation. They concluded that routine use of bone grafting in comminuted forearm fractures is not indicated. Wei and colleagues similarly studied bone grafting of acute forearm fractures. They also found no significant difference in union rate in groups treated with or without cancellous grafting. Neither study investigated significant bone loss, which is uncommon in the forearm.
Ring and colleagues presented a group of patients who had diaphyseal forearm nonunions and segmental defects that were treated with plate fixation and autogenous cancellous grafting. Without a control group, they found that all 35 patients healed within 6 months without subsequent procedures and that they experienced an improvement in upper extremity function.
Barbieri and colleagues reported on the technique of iliac crest bone block grafting to treat forearm nonunions associated with diaphyseal defects in 12 patients. The graft incorporated without additional procedures in 10 of 12 patients. The investigators concluded that the technique of bone block grafting to correct diaphyseal defects of the radius or ulna is relatively easy to perform and has a high success rate.
Similar to the forearm, albeit to a greater extent, the humerus has a very good soft tissue envelope. Although no literature is available on the acute bone grafting of humerus fractures, its use in nonunions is well established. Hierholzer reported the results of treating humeral nonunion or delayed union with open reduction and internal fixation combined with either iliac crest autograft or demineralized bone matrix grafting. Union was achieved in 100% of patients treated with autograft and 97% treated with demineralized bone matrix grafting. The study did not find significant morbidity associated with harvesting at the donor site.
Hsu and colleagues performed a retrospective review of 105 humeral nonunions over 19 years treated with compression plating and autogenous cancellous bone grafting. They found all fractures to have united at an average of 16 weeks, concluding that this treatment option was reliable and effective in humeral nonunion.
The femur has one of the best healing rates in the body, and no studies analyze the use of acute autogenous bone graft in fractures. Correspondingly, the literature on femoral nonunions is sparse and often involves small sample sizes. In the supracondylar region, Chapman and Finkemeier reported excellent union rates with revision open reduction, internal fixation, and autogenous cancellous grafting, with all 18 fractures going on to union. Wang and Weng combined autogenous bone grafting with allograft struts and also achieved union in all 13 patients in their study. The preservation of blood supply using proper surgical technique was stressed in these studies.
Finkemeier and Chapman reviewed a series of patients who had femoral diaphyseal nonunions. Before bone grafting a diaphyseal nonunion, they recommended a reamed exchange nailing, reserving autogenous bone grafting as a second-stage procedure.
The tibia remains one of the most grafted sites in the body for various reasons. It is the most commonly fractured long bone, has a relatively poor soft tissue envelope and vascular supply (especially distally), and is frequently devascularized and contaminated when associated with high-energy open injuries. Many studies have investigated acute bone grafting of tibial fractures; however, because many severe tibia fractures require soft tissue reconstruction, such as free or rotational flaps, the grafting is typically delayed. Once soft tissues have healed, which often occurs in 6 to 8 weeks, the bed is appropriate for bone grafting.
Blick and colleagues investigated the use of prophylactic bone grafting in 53 high-energy tibial fractures over a 2-year period. The patients were matched with historical controls that required bone grafting after a diagnosis of delayed union or nonunion. Patients underwent planned posterolateral cancellous bone grafting on all fractures in the study within 16 weeks. The investigators found a statistically significant difference in time to union between the groups. They recommend posterolateral grafting at 2 weeks in open fractures closed secondarily or those needing only a rotational flap for coverage. In wounds requiring free flaps for coverage, they recommend bone grafting at 6 weeks.
Christian and colleagues analyzed the use of massive amounts of autogenous bone graft in the treatment of open tibial fractures with large diaphyseal defects. Eight patients with grade 3B open tibial fractures were treated with external fixation, soft tissue coverage, and antibiotic bead placement initially. At a planned later date, the beads were removed and a large iliac crest autogenous graft was placed 6 to 8 weeks after coverage of the wound. The fractures healed in all eight patients. The authors note that this option is an alternative to free vascularized fibular graft (FVFG) in patients who have either a single vessel to the leg (and therefore are not candidates for microvascular anastomosis) or wounds worrisome for possible infection. They comment that failed FVFG is more likely to result in amputation, whereas their method provides for easier further attempts at limb salvage.
In a review of management of open grade III tibial shaft fractures, Burgess and colleagues agreed with planned autogenous bone grafting once the soft tissue envelope heals at approximately 4 to 6 weeks. They note that FVFG may be used for large defects but did not specify when this may be most useful.
Yaremchuk and colleagues used a similar protocol for treating osteocutaneous injuries to the leg. They performed scheduled bone grafting once soft tissue wounds were healed at a range of 6 to 16 weeks. Cancellous grafting was performed for defects smaller than 8 cm, and FVFG for those larger than 8 cm. However, the investigators note that the free muscle flap provides an excellent blood supply for bone graft incorporation, and that cancellous grafts may be effective in gaps larger than 8 cm.
In a review of management strategies for tibial bone loss, Watson and colleagues analyzed 50 fractures of the tibia with greater than 50% circumferential bone loss. Cancellous bone grafting was performed in 37 of these patients with good results. The investigators comment that posterolateral bone grafting in these severe injuries can be technically challenging because of disruption or scarring along the interosseous membrane, posing a risk to the neurovascular structures.
In the presence of a free muscle transfer for coverage, the anterolateral approach is easier and safer, and a large, well-covered pocket for graft placement is often present. Another theoretical concern with acute bone grafting is the possibility of graft resorption during the acute healing period, which is often an inflammatory phase. The placement of autogenous graft into an inflammatory tissue bed may result in a resorptive response, which would waste precious autograft.
Furthermore, in open injuries, the risk for infection and graft wastage is also a significant concern. For that reason, some investigators have advocated using allograft and demineralized bone matrix, with or without marrow aspirate, for early grafting with no risk for autograft wastage. Unfortunately, the results of this technique have not been well validated with high-level scientific studies.
The most common condition for tibial grafting remains the tibial nonunion. Bone grafting for tibial nonunion has been described dating back to the early 1900s. Anterolateral grafting was used commonly in the past, but fell out of favor because of poor soft tissue envelope and small area for graft placement. Harmon advocated posterolateral bone grafting of the tibia, which remains the gold standard for treating midshaft and distal tibial nonunion. However, because of the nearby neurovascular structures in the proximal one third of the tibia, the posteromedial approach to bone grafting has been recommended.
From the 1950s through the 1970s, numerous reports discussed the use and location of bone grafting in treating tibial nonunion. In 1955, Jones and Barnett proclaimed success with posterolateral bone grafting of infected tibial nonunions without resecting the fibrous union, and often without treating the infection. In 1966, Hanson and Eppright reported on a similar series with good results. Blair described the use of a diamond-shaped graft wedged between the tibial nonunion site. His idea was novel in that it lacked placement of additional hardware into the wound, although further studies surrounding this technique are lacking.
In 1982, Gershuni and Pinsker analyzed 40 tibial nonunions treated with autograft and cast immobilization. Although they achieved a union rate of 85%, they also experienced significant angular deformity. They recommend treatment with stable internal or external fixation (rather than cast immobilization) because of similar union rates and better functional results. In 1980, Reckling and Waters reported on a large series of tibial nonunions treated with posterolateral corticocancellous bone grafting. They claim that this is a single nondestructive procedure with a high degree of success because the graft is vascularized quickly. They also claim that union can occur in the setting of infection with cessation of the drainage on union.
In 1990, Meister and colleagues reported on 12 years of treating tibial nonunions and delayed unions with autogenous grafting, reaffirming that it is the most effective and reliable treatment available. Simpson and colleagues also reported in 1990 on 30 high-energy tibial nonunions with a 97% union rate after posterolateral autogenous bone grafting, and concluded that “posterolateral bone grafts consistently produced rapid healing of delayed union as well as established nonunion.” Similarly in 1992, Simon and colleagues reported good results with 62 tibial nonunions over an 18-year period treated with posterolateral bone grafting using solid iliac crest, nonvascularized fibula, and cancellous iliac crest. As their treatment algorithm changed over the analyzed period, they used different graft types. They concluded that “cortico-cancellous bone chips resulted in a shorter healing time compared with a nonvascularized fibular graft or a massive corticocancellous bone block.”
In a 1996 review article on tibial nonunion treatment options, Wiss and Stetson commented that autogenous cancellous grafts remained the gold standard in the treatment of tibial nonunion and can be used to treat segments as large as 6 cm with successful outcome in 88% to 95% of cases. Recently, Ryzewicz and colleagues reported on the use of “central bone grafting” for tibial nonunion. This technique involves a lateral approach, anterior to the fibula, and creation of a tibiofibular synostosis. The investigators claim that compared with the standard posterolateral bone grafting, this technique has decreased risk to the neurovascular bundle, the surgical dissection is easier, and the patient can be positioned supine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







