Use of Antibiotics in Bone Cement
Arlen D. Hanssen
Matthew P. Abdel
Introduction
Infection is a devastating complication that occurs in 0.2% to 0.7% of primary total hip arthroplasties (THAs) and 0.95% to 22% of revision THAs (1,2,3,4,5,6). Coventry’s initial three-stage classification of deep infection after hip replacement has been modified to include four different infection categories: (1) Positive intraoperative culture, (2) early postoperative infection, (3) acute hematogenous infection, and (4) late chronic infection (7,8,9,10). The majority of infections are either type 2 early postoperative infections or type 4 late chronic infections.
Prevention of infection includes augmenting the host response, optimizing the wound environment, and minimizing the bacterial load introduced into the surgical wound (11,12). These issues should be evaluated preoperatively, intraoperatively, and postoperatively.
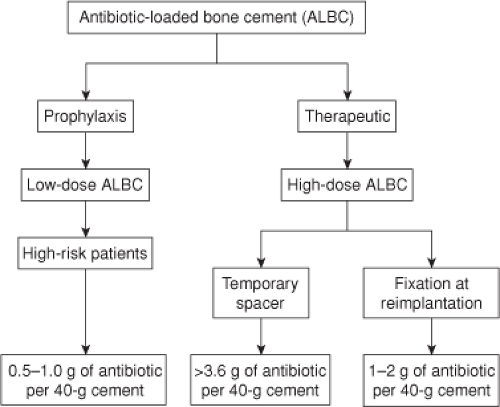
The delivery of local antibiotics to treat musculoskeletal infection has become popular since the concept of local antibiotic delivery by incorporation of gentamicin into acrylic bone cement was first introduced in 1970 (13,14) and antibiotic-loaded bone cement (ALBC) is now the primary method of local antibiotic delivery in orthopedic surgery. When utilizing ALBC during joint replacement arthroplasty, it is essential to define whether the intended use is for (1) prophylaxis or therapy, (2) primary or revision arthroplasty, and (3) low- or high-risk patients (Fig. 97.1). The above factors play an essential role in determining if commercially available or physician-directed ALBCs should be utilized (15). At the current time, a wide assortment of antibiotics can be used with acrylic bone cements, including erythromycin, penicillin, cephalosporins, aminoglycosides, vancomycin, daptomycin, fluoroquinolones, and clindamycin (16,17).
Elution Properties
Elution of the antibiotic from the bone can vary based upon several characteristics, including the type of bone cement, the type of antibiotic being used, its concentration, and whether mixed by hand or prepared commercially (16,17,18,19,20). For instance, antibiotics leach in higher concentrations and for longer periods from Palacos bone cement as opposed to Simplex P, CMW, and Sulfix acyclic bone cements (20,21). Moreover, the antibiotic elution is highly dependent on the porosity of the bone cement and the concentration of antibiotics in the cement (22,23,24). The addition of 25% dextran, for example, increases porosity, which facilitates antibiotic elution (24). The elution is further improved when combining two antibiotics in bone cement (24). As such, some have suggested the minimum combination of 3.6 g of antibiotics (2.4 g of tobramycin and 1.0 g of vancomycin) per 40 g of bone cement powder for effective elution kinetics and sustained therapeutic antibiotic levels (20). Finally, different antibiotics have different elution characteristics, with tobramycin eluting in much higher concentrations than vancomycin (17). However, the elution rate of tobramycin decays much more rapidly than vancomycin (17).
In a novel in vitro continuous flow model, Perry et al. (25) found that the mean percentages of initial antimicrobial release were 11.7%, 14.5%, 6.6%, and 10.9% for tobramycin, gentamicin, amikacin, and vancomycin, respectively. The authors suggest that interchangeability between tobramycin and gentamicin may be cost-effective in humans (25). The same model was utilized to determine that daptomycin
is released from polymethyl methacrylate (PMMA) in a rate similar to that previously determined for vancomycin (26).
is released from polymethyl methacrylate (PMMA) in a rate similar to that previously determined for vancomycin (26).
However, not all antibiotics can be mixed into bone cement given that they must be water soluble, thermostable, and available in powder form (27). The addition of antibiotics dissolved in liquid markedly weakens ALBC, whereas the addition of small amounts of antibiotics in powder form do not substantially weaken the cement (17,28). Furthermore, the antibiotic used should be stable to the heat generated during polymerization, typically 87°C (29). As such, the most commonly used antibiotics are gentamicin, tobramycin, clindamycin, vancomycin, aztreonam, ampicillin, and ofloxacin (3,14,30,31).
Vacuum mixing of commercially available ALBC, compared to mixing under atmospheric pressure, has also been shown to have an effect. Meyer et al. (32) revealed a decrease in antibiotic elution from low-viscosity antibiotic-loaded PMMA products (Cemex Genta), an unpredictable effect for intermediate-viscosity antibiotic-containing PMMA products (Simplex P with tobramycin, SmartSet GMV, and VersaBond AB), and an increase in antibiotic elution from high-viscosity products (Cobalt G-HV and Palacos R+G).
Antimicrobial Efficacy
In addition to elution properties, antimicrobial efficacy depends on several factors. A combination of vancomycin and one of the aminoglycosides provides a broad spectrum of coverage for organisms encountered in deep periprosthetic infections (33,34). In particular, tobramycin has a synergistic effect on the bactericidal activity of vancomycin (33). Alternative antibiotics are utilized when a patient has an antibiotic allergy, the presence of drug resistance, or atypical organisms such as a fungal infection (35,36). For fungal infections, 100 to 150 mg of amphotericin B is typically added to the 40 g of bone cement, in addition to other antibiotics chosen (37).
The preparation of ALBC also appears to have an effect on the antimicrobial efficacy. Squire et al. (38) noted that premixed low- and medium-viscosity ALBCs produced the greatest early bacterial growth inhibition. However, high-viscosity premixed ALBCs appeared to produce superior antimicrobial efficacy on assay day 2. Throughout the remainder of the assay (days 3 to 7), high-viscosity premixed ALBCs trended to superiority over the remaining premixed ALBCs.
Advantages of ALBC
One of the primary advantages of local antibiotics is that they can be administered at much higher concentrations than achievable by parenteral antibiotics, without associated systemic toxicity (16,17,19,33,39,40). Local antibiotic concentrations with local antibiotic delivery vehicles have been shown to reach levels of 3,800 to 4,746 μg/mL (41,42). When tobramycin or vancomycin serum measurements are obtained, serum levels remain at less than 3 mg/L, despite the addition of as much as 3.6 g of powdered tobramycin per 40 g of bone cement (17). In a series of 334 patients receiving ALBC spacers during a two-stage protocol for infected total knee arthroplasties, an average of 3.4 batches of Simplex cement was included with an average of 10.5 g (range, 3 to 16 g) of vancomycin and 12.5 g (range, 3.6 to 19.2 g) of gentamicin. No renal dysfunction was determined by serial monitoring of serum creatinine levels (14).
Furthermore, these high local levels of antibiotics facilitate delivery of antibiotics to avascular areas of the wound that are inaccessible by parenteral antibiotics or resistant to drug concentrations achieved by parenteral antibiotics (14,43). In addition, ALBC, with high concentrations of antibiotics, more effectively treats remaining planktonic and sessile organisms in biofilms (39). Several animal models have noted a decreased risk of infection with ALBC. In a canine experiment, the use of gentamicin-loaded bone cement significantly reduced the rate of implant-related infection, compared with that associated with the use of plain cement (44). This finding was corroborated in a rabbit model in which tobramycin-loaded bone cement was compared with plain bone cement (45,46). Multiple clinical studies, as described below, have indeed confirmed a reduction in deep periprosthetic infections with the prophylactic use of ALBC (13,47,48,49,50,51).
Disadvantages of ALBC
There are several disadvantages of ALBC, particularly when used in low-risk patients or in a prophylactic setting. These risks include inhibited bone healing, degraded mechanical strength of the bone cement, toxicity, allergic reactions, antimicrobial resistance, and increased costs.
Bone Healing
Antibiotics have an effect on fracture healing and bone regeneration which is dependent on the class of antibiotic (52). For instance, quinolones have an adverse effect on fracture healing at low levels achieved with systemic administration (53,54). In an animal model, Huddleston et al. (53) showed significant abnormalities in cartilage morphology and endochondral bone formation with a notable decrease in chondrocytes in animals receiving quinolones compared to controls who received no quinolones. In this same model, there were no effects on fracture healing with the use of cefazolin, gentamicin, or vancomycin (53,54,55). Furthermore, differences between the various antibiotics within the quinolone class have been studied. In an in vitro model, levofloxacin had the least inhibitory effect on cell growth, followed by ciprofloxacin and trovafloxacin. The adverse effect induced by trovafloxacin occurred at a concentration of 0.5 μg/mL, which is lower than serum levels achieved clinically (56).
The in vitro effects of two aminoglycosides (gentamicin and tobramycin) have also been studied. In osteoblast-like cells (derived from human cancellous bone) exposed to media containing various concentrations of gentamicin (0 to 1,000 μg/mL) for 4 days (57), alkaline phosphatase activity was significantly decreased in all cultures with gentamicin at a concentration of >100 μg/mL (57). In a similar study of tobramycin, 400 μg/mL led to significantly decreased cell replication, whereas 10,000 μg/mL caused cell death (58).
Edin et al. (59) investigated the effects of cefazolin and vancomycin on osteoblast-like cells noting that local vancomycin levels of <1,000 μg/mL had little effect on osteoblast replication, but concentrations of 10,000 μg/mL caused cell death. Similarly, cefazolin concentrations of 100 μg/mL had no effect on osteoblast replication, whereas levels of 10,000 μg/mL caused cell death. Such data indicate that vancomycin is safer than cefazolin or aminoglycosides to osteoblasts at higher concentrations (59).
Edin et al. (59) investigated the effects of cefazolin and vancomycin on osteoblast-like cells noting that local vancomycin levels of <1,000 μg/mL had little effect on osteoblast replication, but concentrations of 10,000 μg/mL caused cell death. Similarly, cefazolin concentrations of 100 μg/mL had no effect on osteoblast replication, whereas levels of 10,000 μg/mL caused cell death. Such data indicate that vancomycin is safer than cefazolin or aminoglycosides to osteoblasts at higher concentrations (59).
Mechanical Strength
The mechanical strength of bone cement can be affected by the addition of antibiotics and techniques utilized to prepare the ALBC. It has been shown that >4.5 g of gentamicin powder per 40 g of cement, or the addition of liquid antibiotics, cause a decrease in the compressive strength of bone cement to a level below standards for the American Society for Testing and Materials (ASTM) (60,61). Gentamicin in various concentrations ranging from 0.5 to 2 g per 40 g of Palacos acrylic bone cement has been shown to significantly reduce the shear strength of cement (62). Liquid gentamicin, while bactericidal, substantially diminishes the mechanical properties of bone cement (63). As such, routine use of high-dose antibiotics in cement for fixation of prostheses is not recommended.
There are conflicting reports in the literature on the effects of preparation in regard to mechanical strength of ALBCs. For instance, DeLuise and Scott (64) reported a 36% decrease in strength of hand-mixing generic tobramycin into Simplex P bone cement compared to commercially prepared tobramycin-loaded bone cement and that of plain Simple P cement. However, others have noted no difference in mechanical strength when mixing gentamicin powder into Palacos R bone cement or tobramycin powder into Simplex P bone cement (65




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree