CHAPTER 1 Uni
History and Look to the Future
 The early clinical results with unicondylar implants often included results in which the implants were used to replace the tibio femoral compartments of both condyles with independent components.
The early clinical results with unicondylar implants often included results in which the implants were used to replace the tibio femoral compartments of both condyles with independent components. The use of polyethylene less than 6 mm thick and without metal backing accounted for early failures with the Marmor implant. The FDA now requires a minimum thickness for polyethylene of greater than 6 mm.
The use of polyethylene less than 6 mm thick and without metal backing accounted for early failures with the Marmor implant. The FDA now requires a minimum thickness for polyethylene of greater than 6 mm. Factors that led to higher failure rates of unicondylar implants included younger age, male gender, and most importantly gamma-in-air sterilization. A prolonged shelf age led to oxidative degradation of the tibial polyethylene.
Factors that led to higher failure rates of unicondylar implants included younger age, male gender, and most importantly gamma-in-air sterilization. A prolonged shelf age led to oxidative degradation of the tibial polyethylene. Early failures with unicondylar arthroplasty are related mostly to technical errors with surgical technique and component malposition.
Early failures with unicondylar arthroplasty are related mostly to technical errors with surgical technique and component malposition.Early Clinical Experience with Unicondylar Implants
The earliest nonlinked implants for the management of gonarthrosis were mostly unicompartmental implants often used to replace both the tibial and femoral compartments of the knee. The Polycentric knee was described in 1971 as an implant to restore normal knee movement.1 The probability of success of the first 209 Polycentric implants performed at the Mayo Clinic between July 1970 and November 1971 was 66% at 10 years.2 Results were similar when this implant was used for single-compartment replacement.3 These devices, which were single-radius femoral components, were subsequently abandoned for treating arthritis in both single and bicompartmental arthritis of the knee.
During the same time interval, surgeons were having early clinical success when using the Marmor (Richards, Memphis, TN) knee as a unicompartmental implant. The clinical results, however, did not appear in the literature in a timely fashion. In 1981, Scott and Santore reported early encouraging results with only three revisions in the first 100 patients with a different unicondylar implant.4 Unfortunately, these early encouraging results were overshadowed by inferior results reported by others. In 1976, Insall and Walker had already reported a high failure rate in 19 knees with medial unicompartmental implants of a different design.5 The authors had satisfactory outcomes with 5 lateral unicondylar arthroplasties and related that the use of unicondylar implants in the future may only be indicated for such deformities. In a subsequent report involving many of the same patients, Insall and Aglietti reported 7 conversions to another knee prosthesis and 14 fair or poor results from a group of 22 knees.6 This implant had a coronal curved-on-curved design, and 12 of the 22 cases underwent a concomitant patellectomy. Likewise, Laskin experienced and reported discouraging results in 37 patients with unicondylar implants because of recurrent pain, prosthetic settling, and progression of arthritis.7
Oxford meniscal-bearing implants were introduced a decade after traditional fixed-bearing unicondylar implants. The earliest clinical results were reported in 1986 by Goodfellow and O’Connor on 125 cases with 2- to 6-year follow-up.8 These early cases also were bicompartmental replacements with unicondylar implants similar to the earliest cases with fixed-bearing unicondylar implants. The early revision rate was 4.8% for knees that had intact anterior cruciate ligaments (ACLs). The survivorship for all osteoarthritis knees was 83% at 6 years. In a subsequent study of 301 knees followed as long as 9 years, Goodfellow and O’Connor further emphasized the need to have an intact ACL with meniscal-bearing implants.9 Knees in which the ACL was damaged or absent had a survival rate of only 81% at 6 years. Two hundred five of the 301 cases were bicompartmental arthroplasties. In comparison, Murray et al. reported the outcome of 143 medial unicompartmental arthroplasties in which the Oxford implant was used in knees with an intact ACL.10 In this 1998 report, the survival rate of the implants used as unicompartmental replacements was 98% at 10 years.
Swedish Knee Arthroplasty Register—Early Reports
The Swedish Knee Arthroplasty Register, initiated in 1981, has provided invaluable information as it relates to the outcome of knee arthroplasty surgery and insight into some of the problems that impacted the clinical results. Knutson et al. reported the results of a nationwide survey of over 30,000 knees operated on between 1976 and 1992.11 Total knee components showed gradually improving survival, whereas unicompartmental prostheses did not. The authors reported that this was partly because of newly introduced inferior unicondylar designs that had high failure rates. A survey was mailed to all living patients in the Registry who were operated on between 1981 and 1995 to address the issues of reoperation and patient satisfaction.12 Ninety-five percent of patients answered this survey. Eight percent of patients were dissatisfied. When revision was necessary, the proportion of satisfied patients was higher among patients who underwent a medial unicompartmental knee arthroplasty (UKA) revision than for patients revised following a failed total knee arthroplasty (TKA). Another review of Swedish register data compared the outcome for 699 Oxford (Biomet, Bridgend, UK) UKAs to a matched group of Marmor (Smith & Nephew Richards, Orthez, France) UKAs for unicompartmental arthroplasty.13 After 6 years, the revision rate for the Oxford group was more than two times the revision rate of the Marmor group. Meniscal-bearing dislocation and component loosening were the two main reasons for the 50 Oxford revisions in this cohort of patients.
Unicondylar Arthroplasty in the 1990s
Unicondylar implants fell out of favor among most orthopaedic surgeons during the decade of the 1990s. In 1991, Scott et al. reported that bicompartmental arthroplasties with a condylar prosthesis done in the 1970s had a longer survivorship.14 In this study, the survivorship of 100 consecutive UKAs was 85% at 10 years. Kozinn and Scott also reported very strict criteria for unicondylar arthroplasty to include weight less than 180 pounds, noninflammatory arthritis, an intact ACL, and no evidence of degenerative changes greater than grade II in the opposite and patellofemoral compartments.15 The authors felt that strict selection criteria were essential to avoid failures from progression of disease and failures from implant loosening. Using such strict criteria limited the number of surgical candidates for a unicondylar arthroplasty to less than 5%. Proponents of tricompartmental arthroplasty argued that most orthopedic surgeons in the United States do less than 20 knee arthroplasty cases a year. Therefore, they would only have an opportunity to do 1 or 2 unicondylar procedures a year using strict selection criteria and would have difficulty maintaining the necessary technical proficiency for consistently good clinical results. Furthermore, Padgett et al. related that revision surgery for a failed unicondylar implant was not always a simple procedure.16 In this series of 19 revisions, 76% had osseous defects and two cases required re-revision surgery.
A small number of surgeons advocated UKA for unicompartmental disease and continued to report the benefits of a smaller and less invasive surgical procedure in contrast to full knee arthroplasty. Benefits to the knee with a unicondylar implant included: less blood loss, better flexion in the knee, dominant use of the knee on stairs, and a lesser need for ambulatory aids. Also, patients had better pain relief with the UKA and preferred the UKA to the TKA. Such benefits were reported in studies by Cobb et al. comparing 42 patients who had a TKA in 1 knee and a UKA in the other,17 by Rougraff et al. comparing 120 UKAs to 81 TKAs,18 and by Laurencin et al. comparing 23 patients who had a UKA in 1 knee and a TKA in the other knee during a single hospitalization.19 Knutson et al. had reported earlier from the Swedish Knee Arthroplasty Register data a statistically significant reduction in rate of infection by more than 50% with a unicondylar arthroplasty (0.8% with UKA vs. 2% with TKA).20
The use of unicondylar implants remained sparse through the 1990s. Although patient satisfaction remained high, the revision rates remained marginal. Some of these failures were design issues. As an example, the Robert Brigham implant offered a metal-backed nonmodular tibia that was 6 mm thick. The polyethylene was 4 mm thick. The original Marmor implant had an all-polyethylene tibia that was less than 6 mm thick. Such components had high early failure rates and were withdrawn from the market. As early as 1991, Knutson et al. reported that deformation and loosening occurred in one third of the 6-mm-thick unicompartmental implants placed in rheumatoid knees and one fifth of the osteoarthritic knees within 2 years.21 The 6-mm components had a higher loosening rate. The Food and Drug Administration (FDA) subsequently set greater than 6 mm as the minimum allowable thickness for a tibial polyethylene component. Another design error with unicondylar implants was to try to reduce contact stress by creating a significant coronal curvature to both components. Insall and Aglietti’s original experience featured such a configuration.6 The PCA unicondylar device was a curved-on-curved design in a frontal plane somewhat similar to Insall’s original implant. This implant had an unacceptable failure rate as reported in the Norwegian and Finnish knee arthroplasty registries.22 Positioning the two components correctly in the coronal plane to allow full flexion and axial rotation was technically difficult.
Anderson Orthopaedic Research Institute Results
Four hundred eleven medial unicondylar implantations were performed at the Anderson Clinic between 1984 and 1998.23 The implants were of 12 different designs from six different manufacturers. The Kaplan-Meier survivorship with an end point of revision was 80% at 9 years. Rather than abandoning unicondylar arthroplasty at this time because of this unacceptable revision rate, the risk factors for revision were identified and survivorship reexamined using multivariate data analysis to determine the role (if any) for unicondylar arthroplasty in the treatment of isolated unicondylar arthritis. The risk factors examined were patient factors, including age, weight, and gender, and implant variables, including polyethylene thickness, method of sterilization, shelf age of polyethylene, and implant design. Using Cox proportional hazards regression with revision as an end point, three variables were statistically significant; younger age (p < .01), thin polyethylene (p < .01), and shelf age (p < .01). Of the 411 medial unicondylar knees, 152 had a shelf age less than 1 year and polyethylene thickness of at least 8 mm. The survivorship for this subset of patients was 95%. Confidence was restored in this surgical procedure by using polyethylene of adequate thickness without the potential of oxidative degradation.
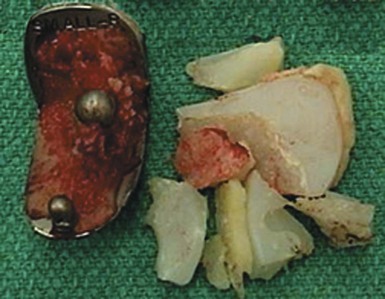
The impact of oxidation on the failure of knee implants is best documented in retrieval studies. The 42 unicondylar implants that were revised at the Anderson Clinic between 1986 and 2000 were cataloged as to reason for revision and then analyzed for wear. Seventy-one percent of the revisions were for polyethylene wear. An analysis of the retrieved components confirmed severe fatigue wear with delamination and in some instances wear-through of polyethylene to the underlying tibial baseplate. No revisions occurred in 42 of the 411 implants that were sterilized by methods other than gamma irradiation in air.23 In another study, Blunn et al. examined 26 retrievals of Marmor unicondylar implants in situ from 1–13 years.24 These nonirradiated tibial polyethylene components showed no delamination. In contrast, Williams et al. in 1998 identified delamination with subsurface white bands characteristic of oxidation in over 80% of gamma-in-air sterilized components.25 In this study, 32 unicondylar implants sterilized by ethylene oxide had no delamination or evidence of oxidation. The impact of shelf age leading to polyethylene oxidation and its impact on survivorship was far greater for unicondylar implants because of infrequent usage of unicondylar implants and the frequent usage and popularity of total knee implants. Implants were manufactured and sterilized in large batches. Depleted inventory was replenished frequently with total knee implants. The shelf age on unicondylar implants at the Anderson Clinic averaged 2.0 ± 1.9 years. This was more than a year longer than total knee implants, which averaged 0.9 ± 1.0 years (AORI Knee Clinic Database). Two studies best demonstrate the impact of shelf age on implant survivorship. In the first study, 100 consecutive SCR (Osteonics, Allendale, NJ) UKA components with an average shelf age of 1.7 years after gamma irradiation in air were divided into two equal groups: shelf age greater than 1.7 versus shelf age less than 1.7 years.26 The survivorship at 6 years was 96% for the shorter shelf-age group versus 71% for the group with the longer shelf age (p < .01). The second study was a review of 75 Duracon (Stryker Osteonics Howmedica, Rutherford, NJ) unicondylar implants (Fig. 1–1).27 Seventy-three of the components had a shelf-age storage of 4.5–6.5 years. Since publication of that study, 65 of the 75 implants were revised in less than 5 years, with all revisions performed for accelerated polyethylene wear.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree